Introduction
Cellular and acellular matrices form a wide-ranging category of advanced wound care products designed to facilitate the natural wound healing process. Examples include bioengineered living cellular and dermal constructs, skin allografts, and a diverse array of collagen matrices derived from animals. Minimally manipulated, intact amnion and chorion offer a newer approach.1 Various methods are employed for making these tissues conveniently available for use, some with a goal of preserving native components including extracellular matrix, growth factors and various soluble mediators. Methods in use include varied washing techniques and ingredients, as well as cryopreservation (frozen ‘wet’ tissue), lyopreservation (lyophilization) and dehydration. Furthermore, final treatment to produce either sterile or aseptic products may include gamma-radiation, e-beam sterilization or no sterilization.
Human placental membranes have been increasingly incorporated into wound care protocols in the United States and globally. These comprise a significant proportion of cellular and acellular matrices used for managing chronic non-healing DFUs. Placental membranes display a range of properties including anti-inflammatory, antimicrobial, anti-fibrotic, and angiogenic characteristics.2,3 A recent review of wound healing with placental membranes4 highlights the processing effects of cryopreservation and dehydration on potential factors available to wound beds. Since Davis5 reported the use of amniotic membrane as superior to xenograft or cadaveric dressings for skin transplantation in 1910, human amniotic membrane (AM) has been used widely in regenerative medicine due to continued discovery of its favorable biological and mechanical properties.6-8 When amnion and chorion are used together, the chorion, which is thicker, is reported to be responsible for 75% of the growth factors present. Amnion with chorion (AC) in various forms have been used for numerous applications, with the primary application of wound healing to date.9,10
The processing of placental membranes, and subsequent retention of soluble cytokines, chemokines and growth factors, depends on the desired application. The BioRetain (BR) method of processing for BR-AC involves gentle disinfection of the membrane, cold isotonic washing buffers, slow dehydration and low dose terminal e-beam sterilization. This process produces a structurally sound, sterile, factor-rich product that preserves the natural integrity of the placental tissue and its components. In this paper, we report on the primary results of the first controlled trial of BR-AC in DFUs.
Methods
Study population
This multicenter, open label, randomized and controlled clinical trial enrolled consenting subjects aged ≥18 years, with diabetes type 1 or 2 and a non-ischemic diabetic foot ulcer (DFU) of 1.0 – 20.0 cm2 graded as Wagner 1 or 2, with a duration of >4 but ≤52 weeks. Patients reported 30+ days of non-healing prior to the run-in. Potential subjects were excluded if their HbA1C was >12%, oral steroid use exceeded 7.5mg daily within the past 30 days, renal dialysis was needed, the ulcer was located over a Charcot deformity, or there was suspicion of osteomyelitis, cellulitis, or other clinical signs or symptoms of target ulcer infection. Full criteria are found on ClinicalTrials.Gov [NCT06511596].
Ethical approval and patient consent
The trial was conducted in accordance with the ethical principles put forth in the 1975 Declaration of Helsinki, and in conformance with the International Conference for Harmonization Good Clinical Practice guideline E6 (ICH-E6), as well as the United States Code of Federal Regulations 45 CFR Part 46, 21 CFR Part 50, 21 CFR Part 56, and 21 CFR Part 312. The informed consent, site and protocol were overseen and approved by a central Institutional Review Board (Castle IRB).
Objectives
The primary objective of this two-arm study was to compare the probability of complete wound healing in each randomized arm over the 12-week treatment period, with healing defined as 100% re-epithelialization without drainage confirmed at two visits two weeks apart. The secondary objectives were to compare differences between randomized arms in days to closure; compare differences between arms in percent change in wound area (cm2) and wound volume (cm3); and, to compare complete wound healing in the SOC arm versus crossed over subjects. Safety was evaluated as the proportion of subjects in each arm experiencing treatment-emergent adverse events (TEAE).
Protocol
A two-week run-in period using SOC allowed the exclusion of potential subjects who achieved 30% or more wound area reduction. Qualifying subjects were randomized to treatment with SOC alone or SOC plus the BR-AC product stratified on wound area at baseline (<5cm2 or ≥5cm2). Healing was assessed by initial visual and physical examination to determine re-epithelialization and lack of exudate, followed by two confirmatory visits, each two weeks apart. Requirements for debridement, wound dressings and off-loading (using Foot Defender (Defender, USA) were standardized. Weekly subject visits included use of an electronic imaging and measurement device (Tissue Analytics (Net Health, USA)) using a standardized protocol to ensure the measurement of the wound surface area and volume was minimally variable. To reduce bias in the determination of closure, one independent blinded medical reviewer reviewed the images in each case where the investigator determined the wound had achieved closure.
Statistical analysis
A Bayesian power analysis was conducted using the R package Bayesassurance, based on a Beta-Binomial model for the proportion of wounds achieving complete closure. Informative Beta priors were specified for each study arm using a synthesis of healing rates reported in 25 studies that supported Medicare coverage decisions for cellular and/ or tissue-based products (CTPs). The resulting priors were Beta (α = 28.61, β = 66.75), reflecting a prior mean healing rate of approximately 30% for SOC alone, and Beta (α = 19.59, β = 23.94), reflecting a prior mean healing rate of approximately 45% for the intervention arm. Simulations were conducted to estimate the probability of trial success, defined as the posterior probability of treatment superiority exceeding a pre-specified threshold of 80%. The results indicated that a sample size of 60 subjects (30 per arm) yields an assurance of 80.25%, meaning there is an 80.25% probability of demonstrating superiority under the specified priors and model assumptions. Randomization used a ratio of 1:1 with block sizes of four.
An interim analysis was planned once 34 subjects completed the study. This analysis was conducted after n=37 subjects had completed the primary endpoint follow-up. The analysis evaluated the posterior probability, and conditional and marginal effects derived therefrom, of primary endpoint success to inform trial adaptations, that is, whether to add subjects to the study if the Bayesian Assurance of 70% was not met.
The final analysis employed a hierarchical Bayesian model to evaluate total wound closure, using a Hurdle-Gamma likelihood. The first, or hurdle component, models whether a wound is completely closed at a given visit. It represents the probability of observing a zero-wound area as a function of treatment arm, visit week, baseline wound size, and random intercepts for subject and site. The second, or gamma component, models the distribution of wound areas for wounds that remain open. This component captures the magnitude of partial healing under the same set of predictors and random effects. Both the hurdle component [logit (ψi) = αψ] and the gamma component [log(µi)=αµ] took into account random effects for site and subject, fixed effect of the arm, fixed effect of time, an interaction effect between arm and time, and the effect of baseline area.
Results
A total of 71 subjects were enrolled at 11 clinical sites in the US (Figure 1). Randomization was by site, resulting in 30 randomized to BR-AC and 41 to SOC alone. Among 90 potential subjects who would otherwise have qualified, 19 were excluded due to progress towards healing in the run-in period, resulting in a ‘hard-to-heal’ study population. Exudating wounds were most often dressed using foam (n=46), followed by alginate (n=31) and hydrofiber (n=5). Overall, 15 subjects did not require any absorbent dressing, while 21 used more than one of these dressing types.
FIGURE 1 PRISMA diagram showing flow of participants through the main trial.
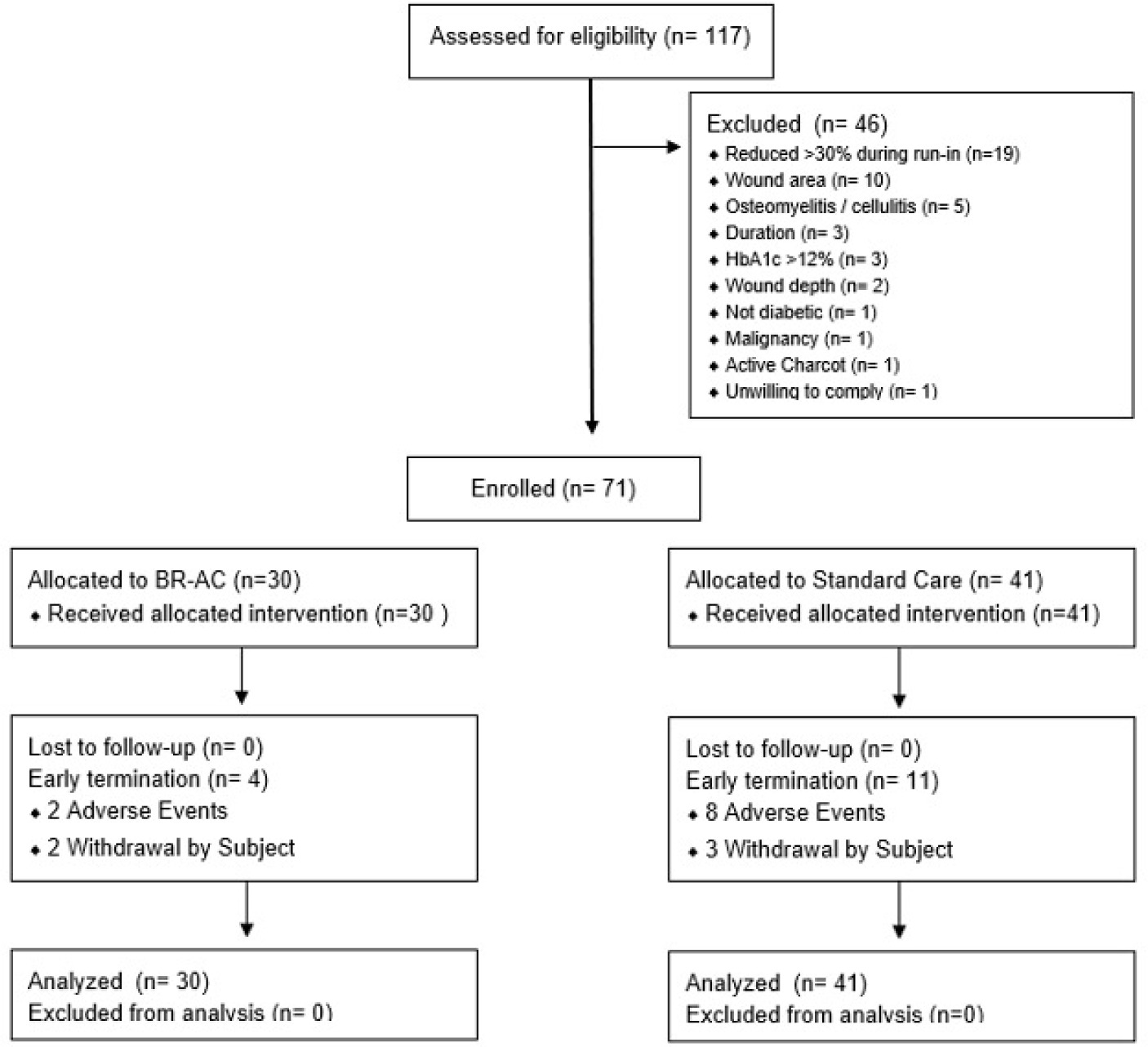
TABLE 1 Demographics of study subjects
| Treatment (n = 30) | Control (n = 41) | All subjects (n = 71) | |
|---|---|---|---|
| Age in years | |||
| Mean (SD) | 56.7±10.8 | 55.1±10.9 | 55.8±10.7 |
| Median | 55.5 | 56.0 | 56.0 |
| Min, max | 36, 76 | 35, 74 | 35, 76 |
| Sex, n (%) | |||
| Male | 23 (76.7) | 29 (70.7) | 52 (73.2) |
| Female | 7 (23.3) | 12 (29.3) | 19 (26.8) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 16 (53.3) | 17 (41.5) | 33 (46.5) |
| Not Hispanic or Latino | 14 (46.7) | 24 (58.5) | 38 (53.5) |
| Race, n (%) | |||
| White/Caucasian | 26 (86.7) | 37 (90.3) | 63 (88.8) |
| Black or African American | 4 (13.3) | 1 (2.4) | 5 (7.0) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 1 (2.4) | 1 (1.4) |
| Other | 0 (0) | 2 (4.9) | 2 (2.8) |
| Diabetes type | |||
| Type 1 | 3 (10) | 1 (2.4) | 4 (5.6) |
| Type 2 | 27 (90) | 40 (97.6) | 67 (94.4) |
SD=standard deviation
In the interim analysis, posterior draws from the observed data at n=37 informed simulation of 1,000 future trials which were assessed for how often the posterior probability exceeded the 95% threshold. The resulting estimate was 91%, which exceeded the pre-specified 70% threshold, indicating that the current sample size provided sufficient power to justify proceeding without further enrollment. At n=37, the probability of healing in the SOC arm was 31.2%, with a 94% credible interval (CrI) of [18.4%-45.3], and the probability of healing in the treatment arm was 59.3%, with a 94% CrI of [41.9%-76.2%]. This translated to a risk ratio of 2.04, i.e. wounds in the treatment arm were 2.04 times as likely to heal as those in the SOC arm. The posterior probability of the superiority of treatment was 97.9%.
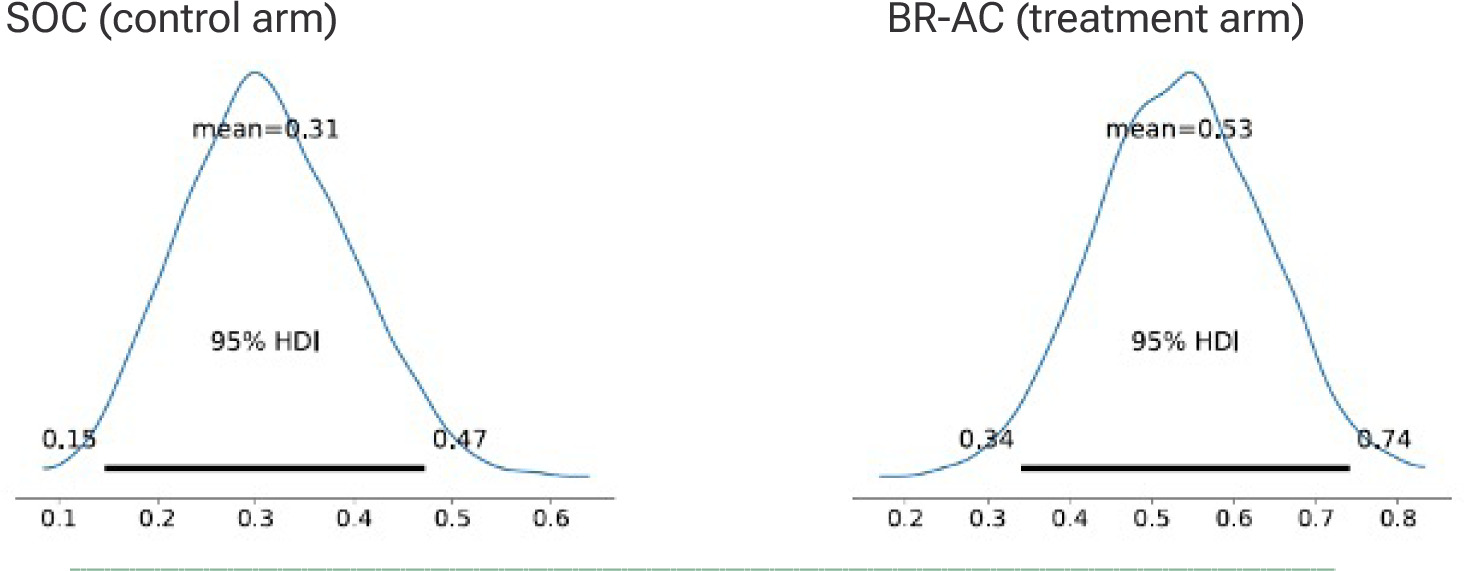
An imbalance in enrollment resulted from using randomization by site, but enrollment continued until the minimum of 30 had been enrolled into the treatment arm. The final analysis of the ITT dataset (n=71) showed 7/41 (17.07%) healed in the SOC arm and 11/30 (36.67%) in the BR-AC intervention arm. For the primary endpoint of healing at week 12, the posterior distributions for wound closure probability showed a clear and clinically meaningful shift in favor of the treatment arm. Taking co-variates into the model, the posterior mean probability of closure was 0.31 (95% CrI: 0.15–0.47) for the SOC arm compared with 0.53 (95% CrI: 0.34–0.74) for the treatment arm. The treatment distribution was centered substantially higher, with limited overlap between intervals (Figure 2).
FIGURE 2 Highest Density Interval (HDI) for posterior probability of closure.
The posterior distributions of the marginal effects at the mean (MEM) for the absolute difference showed a mean absolute difference between treatment and control of 0.22 (95% CrI: –0.01 to 0.45) (Figure 3), with a 96.8% posterior probability that the effect exceeds zero and a posterior mean risk ratio of 1.9 (95% CrI: 0.87–3.2).
FIGURE 3 Marginal effects at the mean – absolute difference
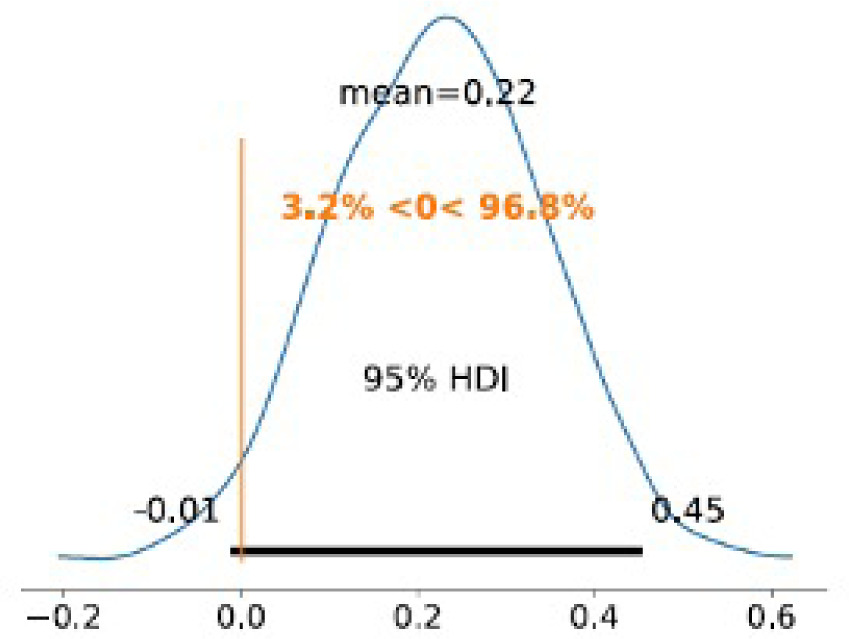
TABLE 2 Target ulcer location
| Treatment (n=30) | Control (n=41) | |
|---|---|---|
| Location (%) | ||
| Dorsal surface | 2 (6.7) | 4 (9.8) |
| Heel | 2 (6.7) | 3 (7.3) |
| Lateral surface | 4 (13.3) | 4 (9.8) |
| Medial surface | 0 (0) | 1 (2.4) |
| Plantar surface | 17 (56.7) | 26 (63.4) |
| Toes (+interdigital) | 5 (16.7) | 3 (7.3) |
| Wagner grade (%) | ||
| 1 | 25 (83.3) | 33 (80.5) |
| 2 | 5 (16.7) | 8 (19.5) |
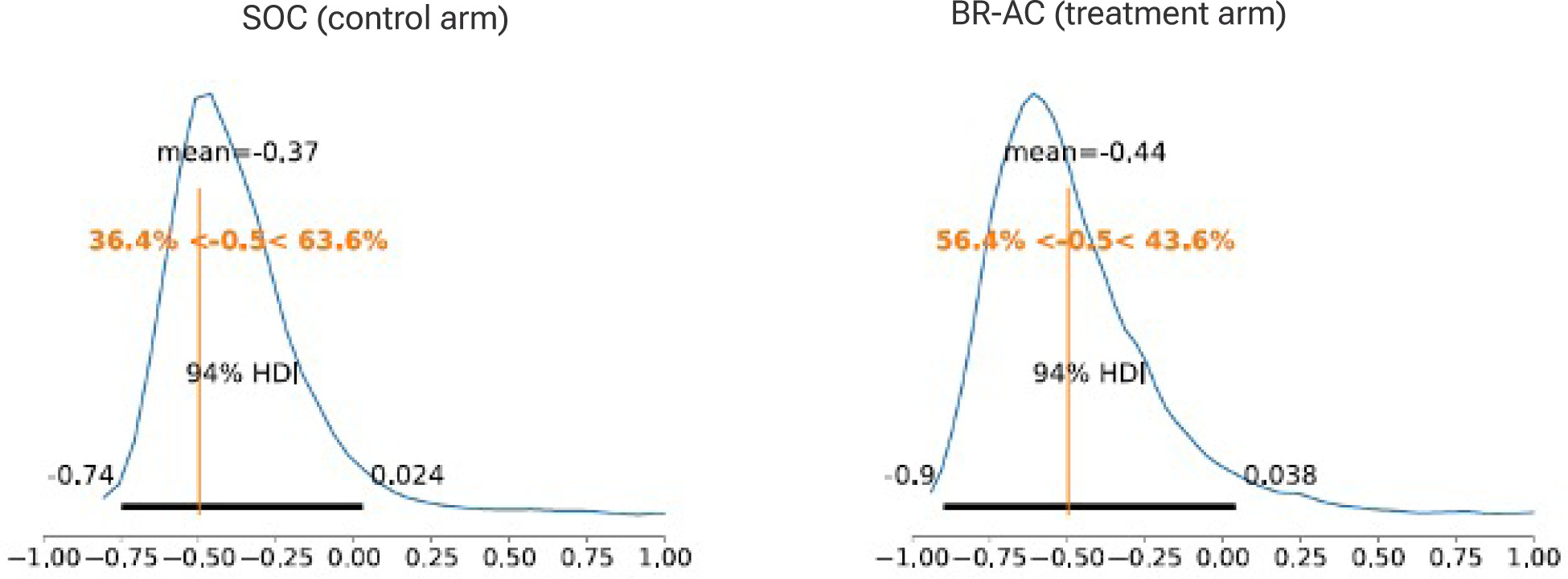
Average marginal effects for percent area reduction for all subjects, healed and unhealed, showed a posterior mean of –0.37 (94% CrI: –0.74 to 0.02) for SOC and –0.44 (94% CrI: –0.90 to 0.04) for treatment (Figure 4), reflecting overall contraction in wound area over 12 weeks. The posterior probability of closing by more than 50% in the SOC arm was 36.4%, compared with a 56.4% posterior probability for the treatment arm (a 20% delta, or 55% increase).
FIGURE 4 Average marginal effects, percent area reduction
Overall, 15 subjects left the study early due to adverse events or simply withdrawal of consent. Between baseline and the 12-week primary endpoint, 20 serious adverse events occurred, 8 of them in three subjects randomized to treatment and 12 in 8 subjects randomized to SOC. Events in the treatment arm were respiratory failure (1), cellulitis (2), diabetic ketoacidosis (1), acute osteomyelitis (1), pulseless electrical activity (1), and sepsis or septic shock (2). None were considered related to the investigational product or protocol procedures. Events in the SOC arm were similar consisting of congestive cardiac failure (2), cellulitis (2), diabetic foot infection (1), hypotension (1), osteomyelitis (4), sepsis (1) and urinary tract infection (1).
Discussion
Effective wound care for DFUs involves comprehensive evaluation of the patient and the wound itself, followed by SOC measures. This includes debridement, infection control, the application of moisture managing dressings, and the alleviation of pressure on high-stress areas. SOC alone typically leaves 70% of DFUs unhealed, even after 20 weeks of treatment.11 The study and use of cellular, acellular and matrix-like products (CAMPs) has resulted in substantial advancements over SOC alone for support of healing in DFU, allowing a greater proportion of wounds to be healed.
The use of the Bayesian approach in quantifying and reporting evidence is regarded as being particularly useful in assisting with decision-making, by accounting for parameters of interest (both random and fixed effects) and using the entire posterior distribution to quantify the evidence.12 Bayesian analysis avoids the dilemma that p-values cannot reveal the plausibility, truth, or importance of an effect.13 As pointed out by Gelman and Stern,14 ‘the difference between ‘significant’ and ‘not significant’ is not itself statistically significant.’ What is more useful is credible evidence of a nonzero effect. In this study, the posterior mean rates of healing with BR-AC were concordant with the prior probabilities obtained from other published DFU trials, with analysis controlling for co-variates in order to discern the responses expected from patients having wounds of the same average location, size, depth and duration. Unlike P values, posterior probabilities can be interpreted directly as probabilities related to the treatment effect.15
The use of a two-week run-in period resulted in a study population reflective of patients with typical wounds needing more than SOC. The rigor of this clinical study was considerable. By controlling or accounting for multiple confounding variables, we have examined the comparison of SOC to BR-AC, and by extension, CAMPs. As strongly evidenced by the findings, BR-AC contributes to improved outcomes in hard-to-heal DFUs over SOC. No safety concerns arose in connection with the use of BR-AC.
The use of a longer run-in period of 4 weeks may have resulted in a harder to heal group of participants, while a longer treatment period may have allowed more subjects in each arm to achieve complete healing. The quality of the healed wounds has not yet been evaluated. However, under the conditions of this commonly used and accepted study design, the accumulated evidence supports a hypothesis of meaningful benefit with use of the tested product.
Conclusion
This is the first controlled DFU trial examining a birth tissue product prepared using the ‘Bioretain’ method designed to retain integrity and contents. With only a 3.2% probability that BR-AC is not superior to SOC, these results provide strong evidence of treatment benefit.