Introduction
Chronic wounds such as pressure ulcers (PUs) impose substantial physical, emotional and financial strain on patients and healthcare systems.1 The development and persistence of PUs are exacerbated by multiple comorbidities: cardiovascular disease, diabetes, renal dysfunction, malnutrition, and immobility, all of which impair healing despite standard preventative and therapeutic efforts.2 Globally, PUs are recognized as a major component of the chronic-wound burden and pose significant strain on healthcare resources.
Advanced wound management incorporates cellular, acellular, and matrix-like products (CAMPs). These products offer multiple therapeutic benefits, including protection of the wound environment, coverage of exposed deep structures, facilitation of surgical closure, and improvements in both functional outcomes and cosmetic appearance.3 A subset of CAMPs, amniotic membranes (AMs), have been demonstrated in previous studies to aid wound closure.4 These biomaterials act as wound covers and are a rich source of amino acids, growth factors and other nutrients that support wound closure.5 AMs are relatively expensive,6 but the material costs are offset if AMs can improve healing in chronic wounds that have not responded to standard therapies, avoiding prolonged treatment and escalating complications.7
This study examined the efficacy of cellular, acellular, and matrix-like products (CAMPs) in achieving complete closure of non-healing PUs that were not responsive to standard of care (SOC) treatment.
Materials and methods
Subjects and data collection
This retrospective, multicenter study included subjects treated for chronic ulcerated wounds, with enrollment initiated in September 2024 and treatments completed in January 2025. Inclusion and exclusion criteria are listed in Table 1. A total of 25 subjects met the study criteria and 14 subjects (56%) had multiple wounds for a total of 37 wounds. The current report is a planned analysis of wound healing progress and parameters only.
TABLE 1 Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Subjects must be at least 18 years of age or older. | The potential subject’s target ulcer is infected or if there is cellulitis in the surrounding skin. |
| The potential subject’s target ulcer must have been present for a minimum of 4 weeks treated with SOC, prior to the initial screening visit. | There is evidence of osteomyelitis complicating the target ulcer. |
| The potential subject must have given permission to share data at the time of treatment. | The potential subject has been treated with hyperbaric oxygen therapy or a CAMP in the 30 days prior to the initial screening visit. |
CAMP=Cellular acellular, or matrix-like Product, SOC=standard of care
Subject data was collected by Sequence LifeScience, Inc. and was deidentified prior to review and analysis, with all subject identifiers removed from the study file. Statistics were calculated using R studio (v. 4.5 software, Austria, 2025). Categorical variables are presented as N and (percentage) and continuous variables are presented as mean (standard deviation) or median (interquartile range).
The Advarra Institutional Review Board determined that this study did not meet the criteria for human subjects research as defined by 45 CFR 46.102(f).
Study intervention and outcomes
Advanced therapy wound care treatment used three-layer placental-derived allo-graft (TLPA; Activate Matrix, Sequence LifeScience, Inc., US). After appropriate wound preparation, TLPA was applied to the wound site according to manufacturer’s instructions. After documentation of wound characteristics, wounds were debrided, and any biofilm, subcutaneous tissue and exudate were removed. TLPA was then applied, with the amount of product used noted. SOC conservative therapies included instructions regarding limb elevation or use of specialized beds/chairs.
Study subjects received advanced therapy wound care treatment for up to 16 weeks, or until complete wound closure (100% area reduction) was achieved. The primary endpoint was complete wound closure of the target ulcer. Secondary endpoints were time (in days) until wound closure, percent area reduction (PAR) in wound size (as measured in cm2) compared to measurement at week 1 (initiation of advanced treatment), number of grafts used, and adverse events associated with the intervention product(s) or procedure(s). Analysis outcomes reported are wound size, PAR, and number of treatment visits.
Results
A total of 25 subjects with 37 PUs met study criteria; 9 were male, and 16 were female, with a mean age of 69.3 (21.5) years. Of the group, 14 subjects had multiple ulcers. The wounds included stage 3 (29.7%) and 4 (70.3%) PUs. The earliest reported onset date of a wound was February 2023, with the latest reported onset date of a wound was December 2024. Patients received a median of 7 (IQR 6-10) weekly applications; the mean was 8.37 (SD 3.5; range 2-16).
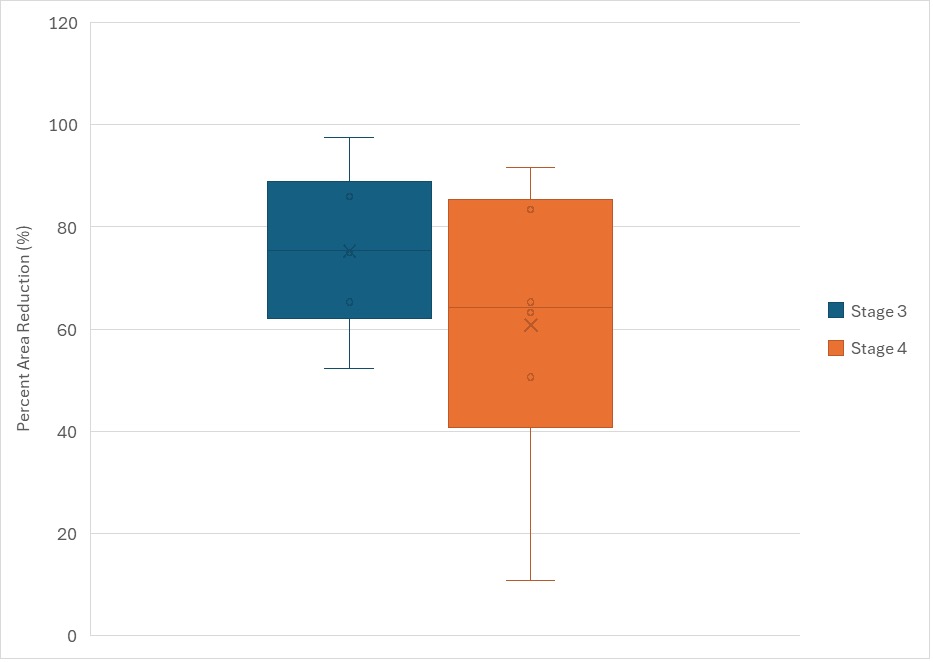
At baseline presentation of the wound prior to initiation of SOC, the minimum wound area was 0.36cm2 and the maximum wound area was 591.36cm2, with a mean of 44.9cm2 (SD 103.5 cm2) During SOC treatment, three (8.1%) wounds achieved 40% or greater PAR. All patients received TLPA during advanced therapy wound care. At initiation (week 1) of advanced therapy wound care, the minimum wound area was 2cm2 and the maximum wound area was 545.16cm2, with a mean wound area of 42.3 (SD 94.5) cm2. The mean amount of product used during advanced wound therapy care was 290.5cm2 (SD 598.9), and the median amount of product used was 88cm2 (IQR 220.4). At the end of data collection 33 (89.2%) wounds achieved greater than 40% area reduction after receiving TLPA treatment. No subjects achieved 100% PAR at or before 16 weeks of TLPA treatment. Figure 1 shows percent area reduction (PAR) during advanced therapy wound care. Stage 3 PUs achieved a mean PAR of 75.8cm2 (SD 15.1) while Stage 4 PUs achieved a mean PAR of 63.3cm2 (SD 22.3).
FIGURE 1 Percent Area Reduction (PAR) for stage 3 and 4 pressure ulcers.
Discussion
In the current study, 25 subjects with chronic stage 3 and 4 PUs refractory to 4-6 weeks of SOC treatment, underwent TLPA treatment for up to 16 weeks. Compared to 8.1% during SOC, at the end of the study, 89.2% of wounds had achieved ≥40% reduction in area during a median of 7 IQR (6-10) weekly applications. These findings suggest that TLPA may substantially enhance closure rates in patients with chronic PUs that did not heal with SOC. However, evidence on advanced biologic and cellular therapies for PUs remains limited compared with other chronic wound types such as diabetic foot ulcers and venous leg ulcers. Given the multifactorial pathophysiology and high recurrence rates associated with PUs, larger, randomized controlled trials are needed to validate these results.
Limitations
These results are subject to all the limitations of a retrospective, observational design. The lack of a control comparison group limits the ability to quantify the degree of improvement achieved with TLPA as what could be achieved with prolonged SOC. Potential confounding factors from comorbidities, primary diagnoses and other sources of unmeasured variance affects the generalizability of the findings.
Conclusions
TLPA treatment was shown to achieve 40% or more wound area reduction in this cohort of 25 subjects with 37 with PUs that had not responded to SOC. These findings suggest that TLPA may serve as an effective adjunctive therapy for managing chronic pressure ulcers, supporting tissue repair and reducing the burden of chronic wound management. Further randomized controlled studies are recommended to confirm these outcomes, evaluate complete closure rates, and determine patient quality of life outcomes.