Executive summary of panel recommendations
| Elimination of Average Sales Price (ASP) in favor of reimbursement range | Replace ASP with a reimbursement range of $478–704/cm2, aligning with clinical effectiveness and economic sustainability. |
| Standardized definition of cellular, acellular, and matrix-like products (CAMPs) | Develop a unified and standardized definition of what constitutes a CAMP. |
| Redraft future Local Coverage Determinations (LCDs) with expanded coverage | Rescind and redraft future LCDs to expand coverage beyond diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs), incorporating real-world evidence into policy-making. |
| Wound care provider taxonomy for CAMPs | Create a taxonomy of wound care providers specifically trained in CAMP utilization, supported by certifications such as Certificate of Added Qualification (CAQ). |
Introduction
The wound care stakeholder community recognizes and values the ongoing commitment to improving quality, value, transparency, and sustainability across Medicare Part B services shown by the Centers for Medicare & Medicaid Services (CMS). Over the past several years, CMS has taken meaningful steps to address longstanding challenges in advanced wound care, including concerns around waste, rising costs, pricing variability, and appropriate utilization. This consensus document is intended to complement those efforts by providing evidence-based, multi-stakeholder recommendations that further strengthen patient access, support innovation, and ensure fiscal stewardship in the coverage and reimbursement of cellular, acellular, and matrix-like products (CAMPs).
As referenced in previous consensus documents such as the Journal of Wound Care (JWC) April 2023 publication, skin substitutes or cellular and/or tissue-based products (CTPs) are now referred to as CAMPs.1 CAMPs represent a broad category of advanced biologics used in acute and chronic wound care, as well as in various surgical specialties, including reconstruction, tendon and dural repair, anastomosis/reanastomosis, and adhesion prevention, among others. Because CAMPs are relatively new and offer a unique set of benefits for healing, there has been an understandable increase in adoption and use for patients who do not respond to older methods of healing.
As of 2025, over 200 CAMPs have entered the U.S. market, each identified by an individual Healthcare Common Procedure Coding System (HCPCS) tied to a branded product. In recent years, CAMPs have come under increased scrutiny from CMS, Medicare Administrative Contractors (MACs), and the Office of Inspector General (OIG) for the United States Department of Health and Human Services (HHS) due to rapid growth in utilization and reimbursement costs. Based on 2020–2024 Medicare claims and a projection to 2025, Medicare spending on CAMPs is expected to exceed $15 billion by year-end 2025, driven largely by rising utilization and Average Sales Prices (ASPs). Although the ASP-based reimbursement model is appropriate for drugs and biologics, its application to CAMPs has proven increasingly ineffective and unsustainable. To address the fraud, abuse and overutilization of high ASP products, CMS has proposed changes to both the Medicare Physician Fee Schedule (MPFS) and Hospital Outpatient Prospective Payment System (OPPS) annual rulings. These proposals underscore the urgency of this consensus document and recommendations by the panel for CMS to consider in rulemaking and fee schedule changes.
Consensus meeting overview
In August 2025, a national meeting was held in Washington, D.C., gathering 32 experts from across clinical, academic, manufacturing, legal and policy sectors to address the clinical, regulatory, and economic landscape of CAMPs. This consensus document summarizes key discussions from that meeting and the panel recommendations are aimed at improving the regulatory and reimbursement environment for CAMPs in the U.S., while concurrently ensuring Medicare beneficiaries have equitable access to advanced therapies for wound healing and amputation avoidance.
Topics discussed
-
History of CAMPs: evolution from traditional skin substitutes to a broader, more complex category of biologic and tissue-engineered products.
-
U.S. Food and Drug Administration (FDA) regulatory classification: key differences in how CAMPs are regulated as HCT/Ps (361 of the PHS Act vs. 351of the PHS Act), biologics, devices, and combinations, and the resulting implications for clinical use and market entry.
-
Audit landscape: increased oversight and audit activity related to CAMPs, particularly in outpatient and Ambulatory Surgical Center (ASC) settings.
-
Proposed unified reimbursement model: recommendations to replace the current product-specific ASP-based system with a more equitable and sustainable model tailored specifically for CAMPs.
-
Impact of future effective LCDs on patient access: how impending Local Coverage Determinations (LCDs) may affect the clinical availability and utilization of CAMPs.
-
The Wasteful and Inappropriate Service Reduction (WISeR) model: introduction of the WISeR model, a proposed prior authorization system that would apply to providers in MAC regions with active LCDs. The model encourages the use of artificial intelligence (AI) in coverage decision support.
-
Need for wound care taxonomy and clinical training: the call for establishing a standardized taxonomy for wound care providers that utilize CAMPs, along with expanded certification and training programs to guide providers in evidence-based application of CAMPs.
This document is focused on U.S. regulatory and reimbursement reforms and is intended to complement international consensus publications such as the 2019 consensus documents "Implementing TIMERS: the race against hard-to-heal wounds,"2 and the subsequent 2023 consensus document titled "Best practice for wound repair and regeneration use of cellular, acellular and matrix-like products (CAMPs)."1
History of CAMPs: clinical use and evidence
CAMPs represent a diverse and rapidly expanding category of advanced biologic therapies primarily used in wound care, surgical applications, and tissue repair. These products typically combine cellular components, such as living or devitalized human or animal cells, with a structural matrix, or scaffold, that mimics the body’s natural tissue architecture to support tissue regeneration, healing, and replacement. Healing from diabetic foot ulcers (DFU) and chronic limb-threatening ischemia (CLTI) should be understood not as cure, but as remission, akin to survivorship in oncology. CAMPs play a vital role in sustaining this remission and preventing recurrence.
CAMPs are derived from a variety of sources, including:
-
Xenografts (tissues from animals, eg porcine, bovine, ovine, and fish)
-
Human and animal-derived amniotic tissues
-
Bioengineered tissues cultured from human cells
-
Cadaveric (allograft) tissues
-
Synthetic or hybrid materials.
The use of placenta-derived tissue in wound care dates back more than 100 years, and clinical evidence supporting the efficacy of CAMPs in both wound care and surgical reconstruction is extensive. A basic PubMed search using terms such as "amnion wound healing", "amnion surgical reconstruction", and "cellular tissue product wound" returns over 2,500 peer-reviewed articles, illustrating the breadth of research on these therapies.
While individual CAMP products vary in terms of clinical evidence, from case series to randomized controlled trials (RCTs), several real-world evidence (RWE) studies have analyzed CAMP utilization at a population level using Medicare claims data. Notably, studies published by Drs. Armstrong et al3 and Tettelbach et al4 consistently found that:
"Medicare beneficiaries receiving standard of care (SoC) plus CAMPs for hard-to-heal wounds demonstrated significantly lower amputation rates, reduced hospitalizations, and improved wound healing times compared with those receiving SOC without a CAMP during the episode of care."
These findings reinforce the conclusion that, regardless of the branded CAMP selected, appropriate integration of CAMPs into clinical practice yields superior patient outcomes in wound healing compared to SOC alone.
Regulatory differences between CAMPs
In the U.S. market, CAMPs are cataloged into three categories: Premarket Approval (PMA), 510k clearance, and 361 HCT/P (Section 361 of the Public Health Service Act). Each pathway group has distinct approval standards, timelines, and marketing claims. Understanding these differences is essential to interpreting the clinical indications, labeling, and coverage policies associated with each product. However, these classifications do not imply that one regulatory pathway is inherently superior to another, nor should each category be interpreted as a ranking of product quality or clinical value.
Historical context and nomenclature evolution
CAMPs have historically undergone multiple naming conventions, including:
-
"Skin substitutes" – commonly used in CMS rulings and HCPCS Level II coding manuals since 2010
-
CTPs (Cellular and/or Tissue-Based Products) – cited in LCDs
-
CAMPs (cellular, acellular, and matrix-like products) – current consensus term reflecting broader clinical and material diversity.
Despite shifts in terminology, the FDA regulatory pathways for these products have remained consistent. The 2020 Agency for Healthcare Research and Quality (AHRQ) Technology Assessment acknowledges the heterogeneity of these products by declining to provide a definition. The agency authors instead explored definitions proposed by other outside sources, FDA pathways, and the – at that time – current list of Q-coded products. Since 2014, CMS has published a table referring to a list of skin substitute products and their respective high or low cost group for outpatient reimbursement in the Outpatient Prospective Payment System (OPPS). However, a lack of an agreed definition of a skin substitute/CAMP has lead to confusion in the market, leaving policymakers to make their own determinations with vague definitions that can confuse a CAMP with a standard dressing. The LCDs attempt to place the burden on the provider to wade through the regulatory information in the patient’s chart to prove the regulatory status of the selected product.
Consensus position
The consensus group acknowledges that, there is an urgent need for a unified and standardized definition of what constitutes a CAMP. A consistent classification system would:
-
Facilitate accurate and equitable coding and payment across care settings
-
Provide clarity for providers in evidence-based decision-making
-
Support transparent and consistent audit and oversight practices
-
Reduce administrative burden in policy making.
Audits of CAMPs: inconsistent interpretations and policy gaps
In light of a significant increase in audits, claim denials, and reimbursement claw-backs related to the use of CAMPs, the consensus group strongly recommends that CMS and/or FDA more clearly define the CAMP category and align audit practices with existing regulatory and coding standards.
Regulatory misinterpretations driving audits
Auditors are increasingly relying on FDA regulatory pathways as the primary rationale for denying or recouping claims, often without clinical or coding context. Several common audit misinterpretations include:
-
361 HCT/P products (such as amniotic membranes) used as a "covering" for wounds are being misclassified as disposable dressings (e.g., gauze or bandages), despite their recognition by CMS as "biologicals," for ASP purposes and alignment with FDA guidance that permits their use as wound coverings under homologous use, and published evidence demonstrating their effectiveness in improving wound healing."
-
510(k)-cleared CAMPs are often misinterpreted as wound dressings, despite the inclusion of HCT/Ps and 510(k) products in CMS rulings describing the "skin substitutes" eligible for reimbursement under the OPPS.
This lack of understanding results in erroneous audits, where claims are denied not because of a lack of clinical merit, but due to the inaccurate categorization of these products.
Ignored coding and coverage evidence
Despite well-established references, auditors often ignore coding guidance that clearly define CAMPs: Auditors frequently disregard well-established CMS coding guidance that clearly distinguishes CAMPs from conventional dressings:
-
Every annual HOPPS Final Rule since 2014 includes a dedicated section and table outlining Q and A codes that CMS classifies as "skin substitutes" (i.e., CAMPs).
-
The HCPCS Level II Coding Book includes a distinct section titled "Skin Substitutes", reinforcing that these products are separately identifiable and reimbursable.
-
The CMS HCPCS Workgroup does not issue Q-codes for conventional dressings. Products such as gauze, foam, and calcium alginates are assigned to a categorical A-code and billed under Durable Medical Equipment (DME) codes, which can be found on the Pricing, Data Analysis and Coding (PDAC) website (www.dmepdac.com).
-
While a few biologic products have historically been assigned A-codes, no product that CMS recognizes and reimburses as a skin substitute under OPPS/PFS is listed on the PDAC site as a conventional dressing. This confirms that CAMPs are not wound dressings.
Ambiguity in coverage criteria: the role of the MPIM
The Medicare Program Integrity Manual (MPIM) states that Medicare covers items and services deemed "reasonable and necessary" but fails to define this phrase beyond general references to "safe and effective" or "appropriate" treatments, leaving this standard open to subjective interpretation by MACs, auditors and even Administrative Law Judges (ALJs). This regulatory vagueness has led to inconsistent application of evidentiary standards and misaligned audit outcomes.
Recently, stakeholders have observed that even when all documentation and coding issues identified in audits are corrected, successful reversals on appeal appear to be increasingly rare. This reflects a growing concern that some Medicare audit programs function less as corrective mechanisms and more as financially incentivized investigations, raising the risk of punitive "ambush audits" rather than fair, evidence-based oversight that protects Medicare beneficiaries' access to therapy. External analyses have highlighted this problem across Medicare audits more broadly, noting the misaligned incentives that reward auditors for denials rather than accuracy or clinical patient outcomes.5
Conflict between real-world evidence (RWE) acceptance and LCD policies
Although the 21st Century Cures Act (2016) explicitly encourages the use of RWE to support regulatory and reimbursement decisions, its intent has not been fully reflected in current coverage policy:
-
In response to public comments on the future effective LCDs, all seven MACs acknowledged the existence of RWE and the Cures Act.
-
However, they collectively stated: "For the purposes of this LCD, this assessment is challenged by the variations of products and study designs such that the most equitable way to evaluate each product’s effect is by the RCT outcomes."
-
As a result, the MACs have elected to accept only product-specific RCTs with a SOC group for a product's inclusion in LCD coverage, excluding real-world studies and retrospective claims analyses.
-
The MACs attempt to define clinical SOC within the Indications and Limitations sections of their LCDs; it is much less clear what they will accept within study parameters.
-
It should also be noted that the MACs utilized RWE in crafting the LCDs' limitations on the number of CAMP applications that will be covered and to define an "episode of care".
Unrealistic evidentiary standards in audit appeals
Some providers have escalated audit denials to the third-level appeal before an ALJ, only to be met by subjective interpretation not reflected in FDA, CMS, or LCD development processes or precedents, which routinely consider manufacturer-sponsored trials, such as:
-
Only NIH-sponsored clinical trials will be considered valid evidence of "safe and effective" use (per a particular judge)
-
Manufacturer-sponsored studies, even if peer-reviewed and published, can be rejected if deemed so by the ALJ.
The reality is that NIH rarely funds trials on specific branded CAMPs, making such a standard unattainable and inconsistent with the broader evidence base used in LCD and regulatory decisions.
Consensus position
The consensus group asserts the following:
-
Auditors must be educated and align their interpretations with established FDA guidance, CMS HOPPS and Medicare Physician Fee Schedule (PFS) rulings, HCPCS standards, and Pricing, Data Analysis and Coding (PDAC) listings.
-
Coverage decisions and audits should consider high-quality RWE, consistent with the goals of the 21st Century Cures Act.
-
CMS should issue audit guidance that differentiates CAMPs from DME wound dressings and discourages inappropriate denials based solely on the regulatory pathway.
Unified reimbursement model for CAMPs
CAMPs are currently reimbursed differently based on the site of care in which they are applied, resulting in significant inconsistencies across the healthcare system. Each CAMP is assigned a product-specific HCPCS A or Q code. Before 2014, all Medicare fee-for-service settings, including hospitals, ASCs, and physician offices, were reimbursed similarly, with separate payment for both the CAMP (per square centimeter) and the CPT procedure for its application.
However, in 2014, CMS implemented a significant change through the Hospital Outpatient Prospective Payment System (HOPPS) final rule, bundling CAMPs into the procedural CPT payment in Hospital Outpatient Departments (HOPDs) and Ambulatory Surgical Centers (ASCs). As of 2025, the bundled CPT reimbursement rate is fixed at around $1,829, depending on the local wage index, regardless of the actual cost of the CAMP used. This policy has led many HOPD-based wound care clinics to limit the use of CAMPs to smaller wounds, where product costs remain below the reimbursement rate, thereby shifting patients with larger wounds to other care settings, such as inpatient or non-facility settings, where reimbursement structures differ. The non-facility place-of-service (POS) settings encompass private physician offices (POS 11), mobile unit (POS 15), home-based care (POS 12), assisted living facilities (POS 13), skilled nursing facilities (POS 31), and nursing facilities (POS 32).
In contrast, non-facility settings continue to receive separate reimbursement for both the CAMP and its application. Starting in 2022, The Consolidated Appropriations Act of 2021 required CAMP manufacturers to report ASP data quarterly to CMS. If a CAMP HCPCS code is not listed on the ASP file, reimbursement defaults to the Medicare Administrative Contractor (MAC) rate or invoice-based pricing.
Over the past five years, the average-weighted ASP for CAMPs has surged from $209.33 to $1,328.54 per square centimeter, with some individual ASPs exceeding $4,500 in recent quarters (Table 1). The rising costs and inconsistencies in reimbursement policies have created access challenges and financial strain across various care settings.
TABLE 1 Estimated Medicare spend on CAMPs by year
| Year | Estimated Medicare spend on CAMPs | Paid average selling price/cm2 | Average selling price file/cm2 |
|---|---|---|---|
| 2020 | $431,921,235 | $163.28 | $209.33 |
| 2021 | $827,983,880 | $258.41 | $331.29 |
| 2022 | $1,276,781,764 | $338.51 | $433.98 |
| 2023 | $3,883,091,207 | $645.74 | $827.87 |
| 2024 | $7,279,429,863 | $1036.26 | $1,328.54 |
Retrospective analyses of Medicare claims confirm that the majority of CAMP-related cost escalation stems not from the broader wound care community, but from a disproportionately small subset of outlier providers. These analyses, covering 2016–2024, stratified providers by National Provider Identifier (NPI), utilization, and total spending; outliers were defined as the top 100 providers by total Medicare expenditure on CAMPs.
In 2024, fewer than 3% of non-facility providers were responsible for nearly half (47%) of total Medicare CAMPs spending, equating to $3.4 billion of the $7.2 billion billed. A detailed breakdown of the top 100 providers shows outsized spending patterns among specific specialties and a heavy reliance on home-based sites of service (Figure 1). Compared with all other providers, these outliers applied CAMPs nearly three times as often per patient (9.9 versus 3.6 applications), used substantially larger grafts (51.6 cm2 versus 12.6 cm2), and drove average episode costs of $541,406 per patient — more than sixteen-fold higher than the $33,544 average for others.
FIGURE 1 Breakout of the highest spending 100 providers in 2024. $3.4 billion was spent by the top 100 represented in these graphs. A) The named specialties represent 79% of the top 100 providers. B) Place of service for the top 100 providers by highest spend in 2024.
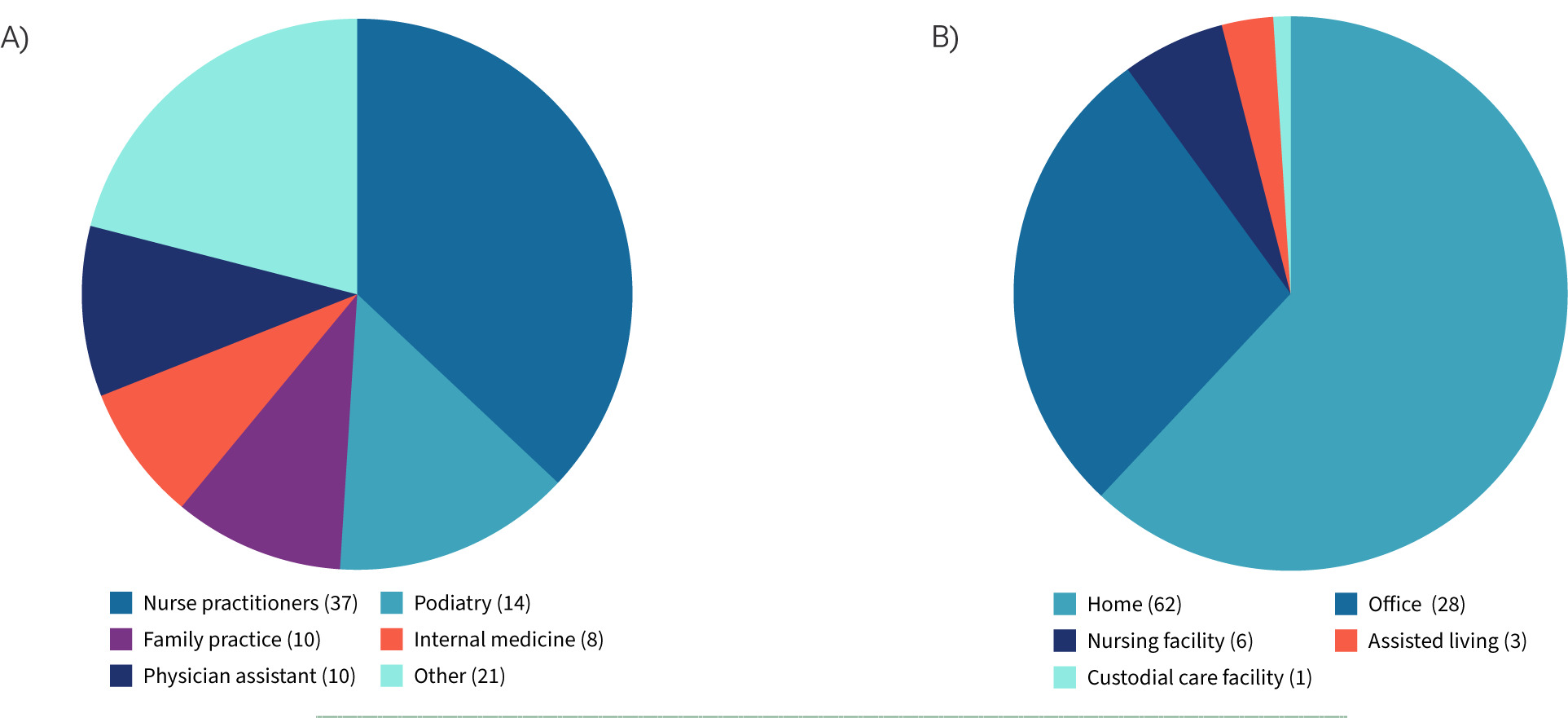
In contrast, the overwhelming majority of providers demonstrated responsible, guideline-concordant utilization. This evidence highlights that blunt coverage restrictions, such as arbitrary application caps or the exclusion of more than 90% of products, risk punishing responsible providers and harming patients, while failing to address the true source of waste: disproportionate billing by a small subset of providers.
In June 2025, CMS issued proposed updates to the HOPPS and the Medicare PFS, signaling a major shift in reimbursement policy for CAMPs. The proposal, published in the Federal Register, would eliminate the longstanding bundling policy in the HOPD setting and establish a uniform payment methodology across all fee-for-service facility and non-facility care settings. A fixed-fee per cm2 reimbursement model at an appropriate payment level, paired with targeted oversight and real-time audit triggers, would offer a more equitable solution that safeguards both access and the Medicare Trust Fund.
Proposed reimbursement models
1. 2026 single-tier payment
CMS proposed a flat payment rate of $125.38 per cm2 reimbursement for all CAMPs, effective in 2026. This rate would apply universally across all HCPCS-coded CAMPs listed in the ASP drug file. The rate is derived solely from Q4 2024 Hospital OPPS claims data and hospital outpatient clinic utilization.
However, stakeholders have raised significant concerns regarding the use of current OPPS package methodology as the determinant for a flat rate across all products. A consensus group of experts agreed this is problematic because:
-
The current ASP-based approach in non-facility settings is not a sustainable reimbursement model.
-
Due to the cost of CAMPs and limitations to bundling, the current HOPD bundled payment system creates barriers to treating larger hard-to-heal-wounds, forcing these wound types to find treatment in non-facility settings.
-
2026 Hospital OPPS proposes to unbundle skin substitutes in the facility setting creating a consistent payment methodolgy across multiple places of service. While is this a significant win (if finalized), the rate must be sustainable to cover the overhead and costs of grafting material.
-
The uniform rate of $125.38/cm2 is artificially low, since this proposed rate is based solely on Medicare claims data from the HOPD setting. The proposal does not use any data from the physician's office or AMWCP settings, where, over the last several years, the bulk of CAMPs use has occurred – especially for the most complex patients with the largest and hardest to treat wounds.
-
A single-tier model must reflect comprehensive data across all care sites to be viable and equitable.
An alternative analysis from the consensus group, based on 2025 projected Medicare spending for CAMPs in post-acute settings ($15.38 billion), supports a more realistic single-tier rate closer to $700/cm2. This rate is derived from reducing the projected spend by 69% divided by the total number of CAMPs units billed per the Medicare claims data. In 2026, this model would reduce Medicare spending by 69%, saving over $10.5 billion annually and more than $105 billion over a 10-year horizon. The group also recommends that this flat rate grow annually with the Consumer Price Index for All Urban Consumers (CPI-U), rather than fluctuate with manufacturer-reported ASPs.
2. 2027 three-tier payment model by FDA classification
For 2027, CMS proposed transitioning to a three-tiered payment structure, assigning different rates based on each CAMP’s FDA regulatory pathway:
-
PMA (Pre-Market Approval)
-
510(k) (Substantial Equivalence)
-
361 HCT/P (Human Cell, Tissue, and Cellular and Tissue-Based Products).
Although a tiered approach has not yet achieved broad stakeholder consensus, CMS has likewise not proposed specific rates for the respective categories. There was a consensus that the statistical differences in clinical outcomes between the three categories should drive the determination of tiered rates, rather than just the regulatory categorization or the level of research performed. In addition, relying solely on hospital outpatient claims is not acceptable, since it omits critical post-acute care data. The consensus group emphasized that rate setting must incorporate volume-weighted claims across all sites of care, combined with Q4 2024 ASP data, to ensure accuracy and sustainability. As with the single-tier model, they recommend CPI-U-based annual growth, independent of ASP fluctuations.
The proposed unified payment methodology marks a major policy shift that could significantly improve equity and access to CAMPs across various care settings. However, accurate and sustainable reimbursement will depend on the inclusion of comprehensive claims data and a stable pricing model tied to economic indices rather than variable ASP inputs.
Impact of future LCDs on patient access to CAMPs and quality of wound care
In November 2024, all seven MACs released identical draft LCDs limiting the use of CAMPs for the treatment of DFUs and VLUs. These proposed LCDs introduce significant restrictions that may adversely impact both patient outcomes and provider workflows. Key restrictions and concerns include:
-
Misinterpreations of RWE: The proposed LCDs cap CAMP application at 8 per wound over a 16-week episode of care, referencing a 2021 Armstrong3 and three separate Tettelbach real-world evidence (RWE) studies to set coverage limitations.6-8 Imposing a 16-week limit does not allow for real world interruption in care which is common among Medicare beneficiaries due to intercurrent medical problems such as heart attacks, stroke, broken hips, pneumonia, etc.
-
Market impact: under these LCDs, only 17 CAMPs would remain covered, down from a market of more than 200 in use today. These LCDs reject RWE as valid evidence for coverage determinations of individual CAMP products, favoring only RCTs. This narrow standard eliminates over 90% of current CAMPs from coverage. This dramatic contraction of covered products would create supply chain challenges, reduce provider flexibility, and introduce clinical delays in care.
-
Cost implications: the proposed policy does not address escalating Medicare costs, as the remaining covered products could re-price their ASPs, under the current payment model, at significantly higher levels, undermining any intended cost-containment goals.
Consensus group recommendations
The stakeholder consensus group strongly opposed the implementation of the future effective LCDs, citing both clinical and operational concerns:
-
Rescind and redraft the LCDs: the group recommends withdrawing the proposed LCDs and reissuing them either as redrafted LCDs or, if this cannot be achieved, as a National Coverage Determination (NCD). This policy should be based on broader clinical evidence, including appropriately interpreted real-world studies such as those by Armstrong et al3 and Tettelbach et al,6-8 which demonstrate that CAMPs reduce amputations, complications, and hospitalizations across wound types.
-
Expand indications beyond DFU/VLU: All hard-to-heal wounds, regardless of etiology, progress through the same healing cascade.9 Therefore, CAMP coverage should be condition-agnostic, with eligibility based on clinical criteria such as wound size, duration, and failure of conservative treatment, in line with Sheehan’s 2003 study showing that wounds failing to reduce in area by 53% in 4 weeks have only a 9% chance of healing without advanced therapy.10 Coverage should also consider the reconstructive use of CAMPs in surgery. The reasonable and necessary threshold that serves in absence of express policy from CMS, is too vague for providers who have been rocked by audits and are fearful of effectively treating wounds and ulcers that do not neatly fit into a DFU or VLU etiology.
-
Simplify and standardize documentation: current and proposed LCDs contain burdensome and ambiguous documentation requirements, leading to inconsistent audits and claim denials. The group recommends creating clear, concise templates that can be integrated into electronic medical records (EMRs) to prompt providers to capture necessary data points efficiently and uniformly. In fact, a well-accepted wound bed management LCD, titled Wound and Ulcer Care (L38904), already exists. Developed by Noridian and effective as of November 28, 2021, this LCD, which can be easily updated, provides guidance on standard wound care applicable to all wound types, not just those that may become candidates for CAMPs. Maintaining two separate LCDs, one focused on wound bed preparation/optimization, and another on CAMP utilization, aligns with the clinical approach to wound management currently practiced by all wound care providers.
The proposed LCDs, as written, represent a step backward in evidence-based care, with potential negative impacts on clinical outcomes, provider operations, and system-wide efficiency. To support both patient access and cost-effective care, CMS should revise its coverage framework to be inclusive of high-quality real-world data, clinically guided thresholds, and supportive of modern care delivery models.
WISeR model and its impact on CAMPs
The WISeR model, proposed by CMS in 2025, is a cost-containment initiative designed to curb overutilization of services deemed clinically inappropriate or wasteful based on utilization data and clinical guidelines. CAMPs are among the services potentially impacted by the WISeR Model, which plans to test the use of enhanced technology (AI) to review provider documentation against active LCD criteria, issuing prior authorizations (PAs) for procedures utilizing a CAMP.
Providers and suppliers for people with traditional Medicare in selected regions will have the choice of submitting a prior authorization request for the CAMP or going through a post-service/pre-payment review. Those who choose the prior authorization route may either submit the prior authorization request directly to a WISeR model participant or through their MAC, which will forward the request to the model participant. Model participants will be held accountable for improving provider and supplier experience, which will be assessed by a survey of questions about the ease of the prior authorization process. If providers or suppliers opt not to submit a prior authorization request for an included service, their claim will be subject to medical review to ensure the delivered service meets Medicare coverage, coding, and payment criteria prior to payment. Providers and suppliers with demonstrated records of compliance may be exempt from the WISeR review process in the future. This exemption, or "gold card," would reduce administrative burden while allowing participants to focus their resources on providers and suppliers at higher risk of delivering unnecessary care.
Despite CMS’s expressed intention to advance the WISeR pilot, its future implementation may be in jeopardy.
The House Appropriations Committee recently advanced the FY2026 Labor-HHS-Education spending bill with an amendment blocking funding for the program.11 While the pilot was limited to six states, stakeholders have warned that the additional delays introduced by mandatory prior authorization could significantly disrupt timely access to CAMPs for Medicare beneficiaries in those regions. This congressional action highlights broader concerns that the risks to patient access from WISeR may outweigh its intended cost-containment benefits.12 Targeted monitoring of outlier providers with disproportionate CAMP billing would be a more effective approach to reducing Medicare expenditures, without imposing broad barriers that restrict patient access and compromise outcomes for Medicare beneficiaries.
Consensus stakeholder concerns
The WISeR framework, if applied rigidly, risks:
-
Delaying treatment for complex wounds • Creating access barriers for underserved populations
-
Inappropriate application of LCD criteria to acute and reconstructive procedures
-
Increasing overall healthcare costs through complications, amputations, or hospitalizations due to under-treatment
-
Inappropriate denials due to the fact that LCDs can be revised by any MAC, and if a universal AI or algorithmic program used for document review is not updated to reflect these changes, which can vary from MAC to MAC, such as updates to regulations or terminology, errors may occur, leading to inappropriate claim denials or approvals and increased compliance risk for providers
-
Creating impediments to treating certain wound types, as current LCDs cover only DFUs and VLUs.
If the WISeR Model pilot reaches fruition, the consensus group requests that CMS publish the following public information for inspection:
-
The underlying algorithm used to make coverage decisions for Pas
-
A list of the most commonly denied pre/post claim review rationale to allow other providers to learn gold standard documentation requirements.
-
In addition, reviewers must be held accountable to strict PA turn-around times which should also be published.
Taxonomy
Healthcare provider taxonomy is primarily used in the National Provider Identifier (NPI) system in the U.S., maintained by the National Uniform Claim Committee (NUCC). It ensures a consistent way to identify and categorize providers in electronic health records (EHRs), insurance claims, and regulatory reporting. This is particularly important in light of HHS findings of providers such as psychiatrists and neurologists administering CAMPs in the mobile setting.
Healthcare Provider Taxonomy is a standardized classification system that categorizes healthcare providers based on:
-
Provider type (e.g., individual or organization)
-
Classification (e.g., physician, hospital, physical therapist)
-
Specialization (e.g., cardiology, dermatology, pediatrics)
Common uses of taxonomy
-
NPI registration: providers select taxonomy codes when applying for an NPI
-
Insurance claims: payers use the taxonomy to understand what services the provider is qualified to perform
-
Credentialing and auditing: ensures that providers are operating within their scope of practice
-
Analytics and reporting: used for categorizing providers in datasets.
Consensus taxonomy recommendation
To date, there is no dedicated taxonomy classification for wound care providers within the current national provider taxonomy system. The consensus group strongly recommends the development and implementation of a distinct healthcare provider taxonomy specifically for wound care.
This new taxonomy would:
-
Recognize wound care as a distinct specialty
-
Enable clearer identification of qualified wound care providers
-
Open a pathway for ACGME-approved standardized training and eventual formal wound care Board certification
-
Improve oversight and quality assurance in the delivery of wound care services
-
Align payer reimbursement policies with appropriate taxonomy and scope of patient care and treatment.
In conjunction with establishing this taxonomy, the group recommends restricting CAMP billing and coding to providers who are formally recognized under this wound care taxonomy or holding another qualifying certification.
To further support the safe and effective use of CAMPs, the consensus group also recommends establishing the standardized certification and education pathways, such as a Certificate of Added Qualifications (CAQ) in wound care, as currently offered by the American Board of Wound Healing (ABWH) and endorsed by the American Professional Wound Care Association (APWCA). This pathway would promote a baseline level of training and clinical competence necessary for the proper utilization, documentation, and application of CAMPs. Integrating a dedicated wound care taxonomy into reimbursement policy would ensure that CAMP billing is restricted to appropriately trained providers, thereby reducing audit disputes, improving payment accuracy, and safeguarding Medicare expenditures while protecting patient access. Further detail on taxonomy and certification will be developed in a future consensus publication.
Final conclusion
The evolving landscape of CAMPs presents both challenges and opportunities for U.S. healthcare policymakers, providers, and payers. While CAMPs offer demonstrable clinical benefits across a range of wound care and surgical applications, the current regulatory, coding, and reimbursement frameworks are outdated, inconsistent, and increasingly misaligned with both the science and clinical practice.
The consensus group of clinical, academic, industry and policy experts is aligned and calls for immediate, systemic reform. These steps include:
-
Establishing a unified definition and classification for CAMPs
-
Adopting a reimbursement model that is sustainable, equitable, and reflective of all care settings
-
Creating a dedicated wound care provider taxonomy to ensure appropriate use and oversight
-
Requiring CAMP-specific training, either integrated into existing accredited educational programs or through a distinct certification pathway, with both approaches recognizing the Certificate of Added Qualification (CAQ) in wound care as the benchmark for provider competency
-
Revising restrictive and clinically flawed Local Coverage Determinations (LCDs) that jeopardize access to care, compromise patient outcomes, and dismiss robust real-world evidence.
LCD restrictions and a poorly calculated flat-rate per cm2 pricing disproportionately harm underserved populations, rural hospitals, and safety-net clinics. Data show that <3% of providers account for nearly two-thirds of CAMP-related Medicare spending, underscoring the need for targeted oversight rather than broad restrictions.4 Consensus modeling supports a reimbursement range of $478–704/cm2, aligned with both clinical effectiveness and economic sustainability.4 This harmonizes with the proposed $700/cm2 benchmark while providing flexibility grounded in real-world cost structures.
Audit practices must also align with regulatory intent, rather than subjective misinterpretations of FDA pathways, and incorporate both real-world data and FDA-reviewed clinical evidence. Furthermore, CMS’s WISeR model and any prior authorization programs must prioritize transparency, efficiency, and equity to avoid delays in care and unnecessary administrative burdens.
Ultimately, the responsible integration of CAMPs into modern healthcare requires a regulatory and reimbursement ecosystem that is transparent, clinically grounded, broadly evidence-based and structured to support innovation while safeguarding the Medicare Trust Fund. The consensus group urges CMS, FDA, Congress, and other stakeholders to adopt these recommendations to protect patient access, reduce system inefficiencies, and support evidence-based wound care in the U.S.