Introduction
Around 10.5 million Medicare beneficiaries were affected by chronic wounds in 2019.1 A chronic wound is generally defined as any wound that fails to heal within a reasonable timeframe. Chronic wounds can be categorized into the following four groups: arterial, pressure, diabetic, and venous. The recurrence rate for diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs) are estimated to be between 60-70%.2 Despite the well-established standard of care for chronic wounds, which includes sharp debridement, offloading, and maintaining proper moisture balance, a notable gap remains between historical outcomes and desired results.
Globally, more than 500 million people are affected by diabetes, with the majority suffering from type 2 diabetes.3 DFUs occur in 1-4% of individuals with diabetes and the presence of a DFU carries a 42% mortality rate within 5 years.4,5 Causes of DFUs include increased pressure on weight-bearing foot areas, friction from ill-fitting shoes, gait abnormalities, and sustained injuries.6 The evaluation and classification of DFUs often consider factors such as size, depth, severity of infection, presence of peripheral neuropathy or peripheral artery disease, and their location.7 The central strategy in managing DFUs is prevention, achieved through regular screening for complications in diabetic patients. This often requires a blend of primary and specialized care, which can be costly.8 The treatment expenses for DFUs in the United States surpassed $358 million dollars in 2019.9 Poor outcomes are more likely for patients with a low socioeconomic status. Furthermore, chronic wound treatment receives inadequate financial support. Despite a total funding allocation of over $7 billion dollars by the National Institutes of Health from 2002 to 2011, only 0.17% was directed towards research on DFUs.10
An estimated 4.5 billion individuals globally were affected by chronic venous disease stages C1-C6 in 2020, and approximately 0.1-0.3% of the world’s population developed a VLU.11 Risk factors for the development of a VLU include non-compliance with compression therapy, lower extremity orthopedic procedures, obesity, and a history of deep vein thrombosis.12,13 Approximately 7% of VLUs are estimated to remain unhealed after 12 months,14 and VLUs have a recurrence rate exceeding 70% after they are closed.15 As with DFUs, treatment of VLUs focuses on prevention. Low-income individuals, especially those from minority communities, often face difficulties in accessing advanced wound care centers.16 In 2022, the United States faced an estimated annual economic burden of over $4.9 billion for treating VLUs, covering costs related to healthcare practitioners, wound care products, inpatient hospitalization, medications, and compression therapy.17
A promising new therapy for treating non-healing wounds are cellular, acellular, and matrix-like products (CAMPs), which encompass ’A broad category of biomaterials, synthetic materials, or biosynthetic matrices that support repair or regeneration of injured tissues through various mechanisms of action’.18 The application of CAMPs in chronic wound treatment provides several benefits, including creating a protective environment for healing, covering deep structures, aiding in surgical closure, improving functional outcomes, and enhancing the wound's appearance.18
This study employs a novel modified platform clinical trial design. During the COVID-19 pandemic, the need for platform trials became apparent to assess various treatments for a single disease concurrently. These trials can incorporate new treatment arms as they become available. Now used in many areas of medical research, the platform trial design is among several master trial designs. Other designs include the basket design, testing one treatment across various diseases, and the umbrella design, which addresses multiple treatments for a single disease. The platform trial design for this study will be adapted to evaluate various treatments for multiple wound types – DFUs and VLUs. These wounds, though resulting from different pathologies, follow the same healing process. This study will evaluate five CAMP products and Standard of Care (SOC) versus SOC alone in the closure of non-healing DFUs and VLUs. The initial plan is to evaluate the five CAMPs identified in this protocol; however, the platform design allows for the inclusion of additional products which will be added following an interval analysis and accompanied by a protocol amendment.
Study objectives
Primary objective
To determine the between-arm difference in the proportion of subjects achieving complete closure of non-healing DFUs and VLUs with multiple CAMPs plus SOC versus SOC alone over 12 weeks using a modified dual platform (Matriarch) trial design (Figure 1). The platform design allows for the inclusion of additional products by modifying the protocol.
FIGURE 1 Design of the STABLECAMP Matriarch trial
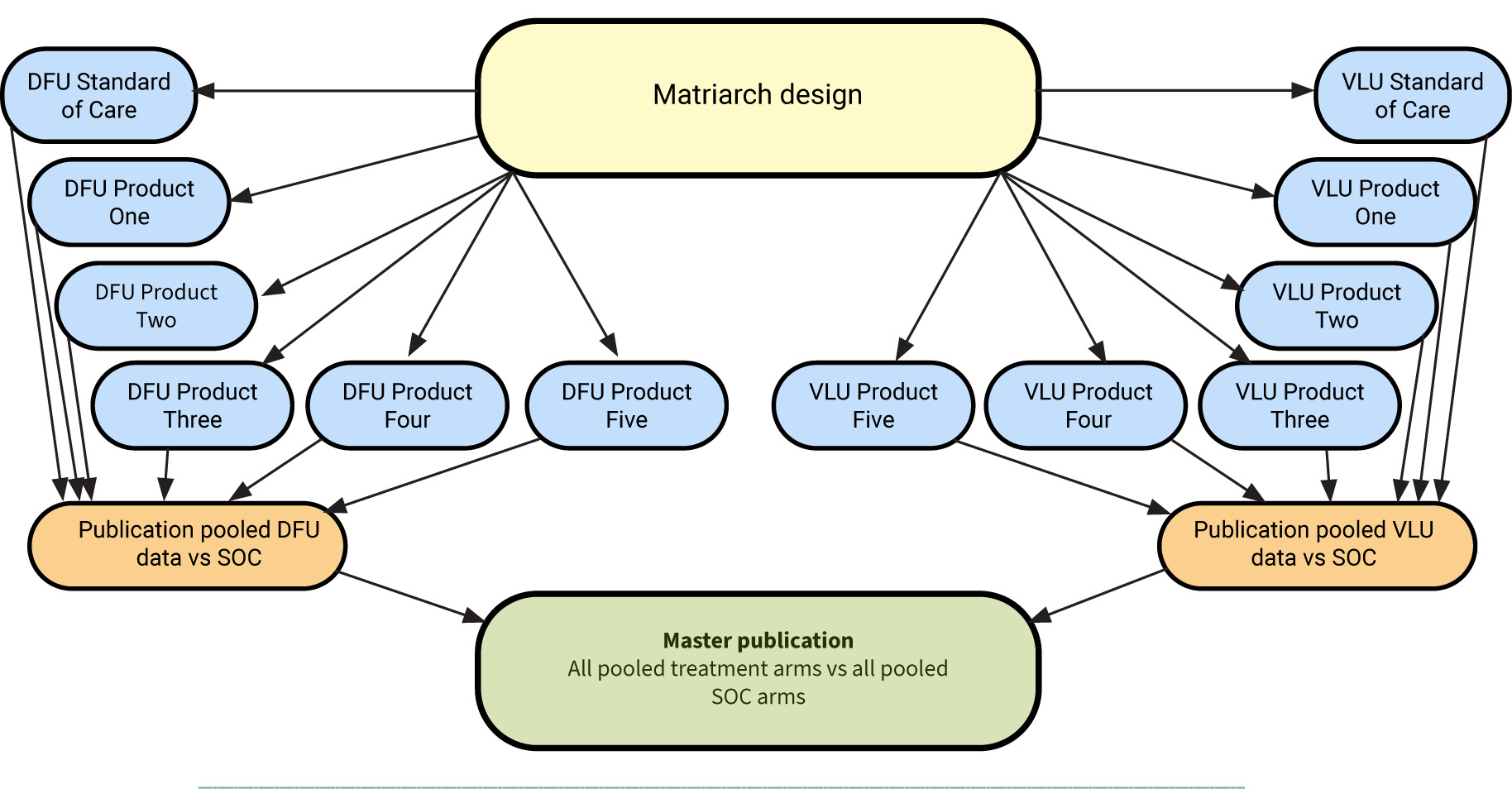
Secondary objectives
-
To determine the between-arm difference in the time to closure over 12 weeks for CAMP plus SOC versus SOC alone.
-
To determine the between-arm difference in the percent area reduction (PAR) at weekly intervals for CAMP plus SOC arms versus SOC alone.
-
To determine the between-arm difference in pain for patients who present with a VAS score of greater than 4.
-
To evaluate the between-arm difference in the frequency and nature of adverse events in subjects receiving CAMP plus SOC versus SOC alone.
-
To evaluate the between-arm difference in the quality of life for subjects receiving CAMP plus SOC compared to SOC alone using the Forgotten Wound Score (FWS) and standard Wound Quality of Life (wQOL) questionnaires at treatment visits 1, 4, 8, and 12. Forgotten Wound Score (FWS) and standard Wound Quality of Life (wQOL) questionnaires at treatment visits 1, 4, 8, and 12.
Exploratory objectives
-
To determine compliance with off-loading using digital technology and the effect on wound closure.
-
To determine the proportion of ulcers that close in patients 65 years or older for CAMP plus SOC versus SOC alone.
Material and methods
This study currently includes five products: dual layer, amniotic membrane allograft (DLAG; AmnioCore, Stability Biologics™, LLC, San Antonio, TX, USA); three layer, amniotic membrane allograft (TLAG; Amnio Tri-Core, Stability Biologics™, LLC, San Antonio, TX, USA), four layer, amniotic membrane allograft (FLAG; Amnio Quad-Core, Stability Biologics™, LLC, San Antonio, TX, USA); dual layer, amnion/chorion membrane allograft (DLACG; AmnioCore Pro, Stability Biologics™, LLC, San Antonio, TX, USA); and three layer, amnion/chorion/amnion membrane allograft (TLACG; AmnioCore Pro+, Stability Biologics™, LLC, San Antonio, TX, USA) shown in Figure 2. Each is comprised of donated human tissue intended for homologous use as a barrier and applied as a covering to offer protection from the surrounding environment. The amniotic membrane base provides essential growth factors to aid in wound closure. The products are packaged sterilized in an inner peel pouch, and then placed within a non-sterile outer peel pouch, within a carton. Donor eligibility determinations, recovery, processing, storage, testing, and distribution are performed in accordance with 21 CFR Part 1271, applicable state, and AATB regulations.
FIGURE 2 Image of amniotic membrane allograft products

Study design
Overview of study design
This study uses a modified master trial design where two platform trials will be conducted in tandem; we have coined the term “Matriarch” for this modified design. A detailed trial design flow diagram is found in Figure 3. This design will be used for both DFU and VLU subjects.
FIGURE 3 Flow diagram for the STABLECAMP trial; the same design will be used for each wound type. PI, principal investigator; HCV, healing confirmation visit; EOS, end of study
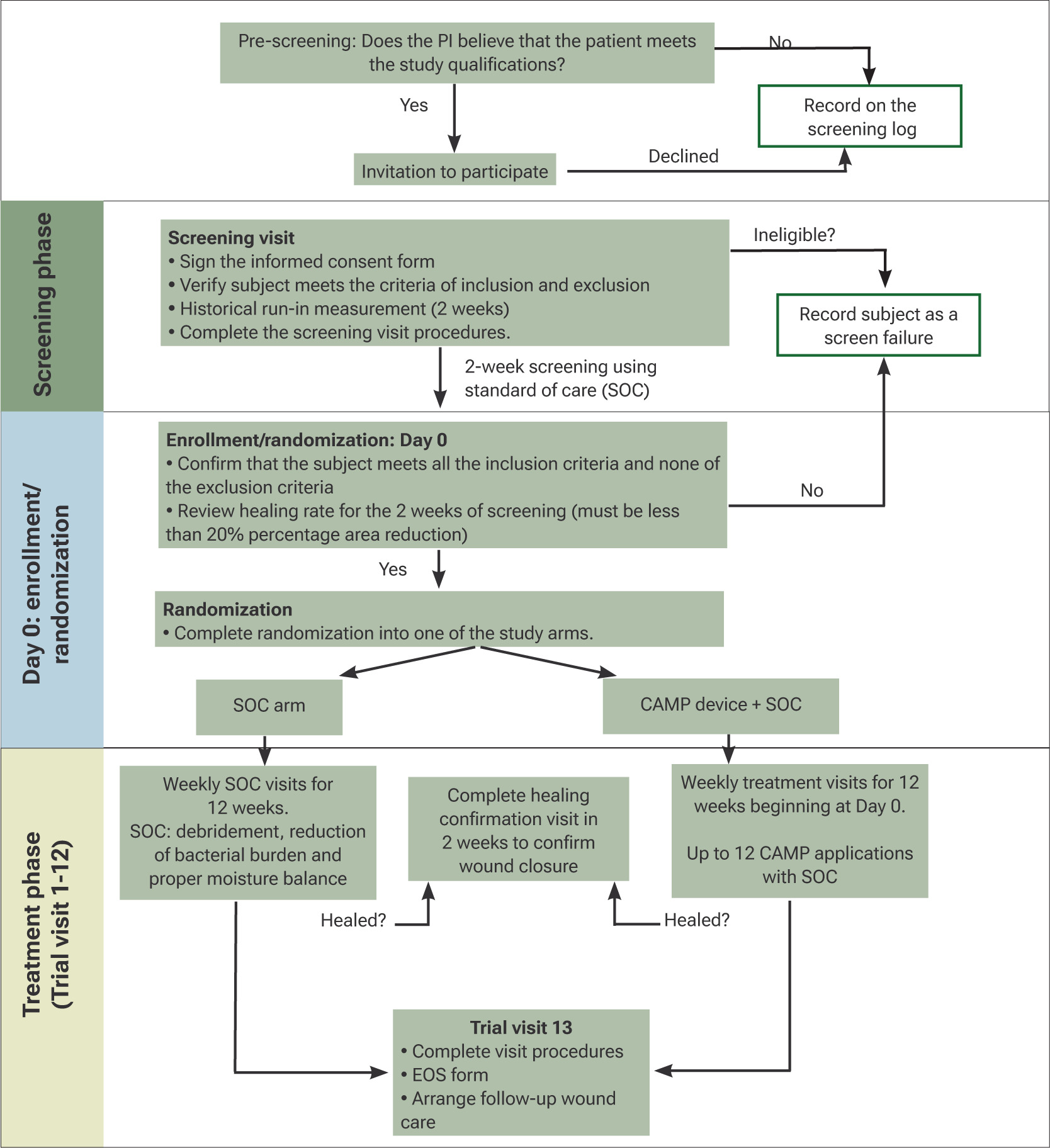
Study duration
It is anticipated that 24 months will be required to complete the evaluation of the first set of CAMPs results. Consistent with the master trial design, this trial does not have a defined end date. Additional products may be added by amending the protocol. The minimum anticipated recruitment rate is 1.5 subjects per site per month, across up to 50 SerenaGroup® or affiliated sites.
Evaluation criteria
Clinical endpoints
The primary clinical endpoint will be the percentage of target ulcers that achieve complete wound closure within 12 weeks.
The secondary clinical endpoints are as follows:
-
The time to healing of the target ulcers over 12 weeks.
-
Percentage wound area reduction from trial visit (TV)-1 to TV-13 measured weekly with digital photographic planimetry, using digital imaging, and physical examination.
-
The number of product- or procedure-related adverse events.
-
Change in pain in the target ulcer assessed from TV-1 to TV-13 using the VAS scale.
-
Change in quality-of-Life based on the wQOL and FWS [time frame: TV-1, TV-4, TV-8, and TV-12/final visit].
-
Number of grafts used.
The exploratory endpoints are as follows:
-
Compliance with a prescribed offloading boot is measured as % of time wearing the boot as determined by digital Blue tooth technology.
-
Percentage of target ulcers achieving complete wound closure in 12 weeks for subjects 65 years of age or older.
-
For VLU subjects only - changes in functional ambulation based on the Functional Ambulatory Category Scale (FACS).
Study population
The study population will be drawn from patients with DFUs and VLUs attending wound clinics for treatment of diabetic foot ulcers or minor amputation wound sites. These subjects will be drawn from the general population.
Sample size
SerenaGroup® performed an extensive literature search to determine the optimal enrollment for the targeted statistical power of this platform trial. For a platform design, the allocation ratio is equal to the square root of the number of intervention groups. This trial will include five (5) intervention groups and one (1) control group, per wound type. The study will be initiated with 3 DFU study products and 1 DFU control group, and 2 VLU study products and 1 VLU control group. If additional arms are added (as allowed by the platform trial design) they will enter after the completion of the initial 5 planned treatment arms. Therefore, to maintain a treatment effect of 0.35, significance level of 5%, 80% power, a 1.4:1:1 ratio will be used for the VLU platform and 1.7:1:1:1 ratio for the DFU platform.
With a total sample size of 324 patients enrolled, the VLU control arm will have 56 patients, the DFU control arm will have 68 patients and the intervention groups will have 40 patients each (200 total). Based on the results of pre-planned interim analyses, the sample size requirements may be re-estimated and adjusted to maintain sufficient study power. The number of subjects may also be increased if additional products beyond the 5 per wound type currently planned are added to the trial as permissible by the modified platform design.
This sample size calculation does not include replacing drop-outs. Dropouts will be included in the intent-to-treat population but may not be included in the per protocol analysis.
Subject recruitment
Subjects will be recruited from the investigators’ clinical practices within the participating wound care clinics and the general public. IRB-approved social media advertising may be used to recruit potential subjects.
Subject screening
Subject screening will be conducted to ensure that subjects meet all the study inclusion criteria and none of the exclusion criteria. Documentation will be required to confirm the ‘historical’ run-in period measurement. A subject is excluded if the surface area of the target ulcer has reduced in size by more than 20% in the 2 weeks prior to the initial screening visit, based on calculating surface area using the formula length X width.
Prior and concomitant therapy or medications
Wounds treated with CAMPs, also defined as skin substitutes, or hyperbaric oxygen therapy (HBOT) in the month prior to screening are excluded. The concomitant use of other CAMPs or HBOT treatments is also prohibited during the trial. Patients taking greater than 10 mg of prednisone daily are excluded. Additionally, if the patient is taking medications that the principal investigator (PI) believes could interfere with wound healing (e.g., chemotherapy, biologics), the patient will be excluded from the trial. Patients taking hydroxyurea are excluded from the trial.
Rescue medications
There are no rescue medications for this study.
Inclusion/exclusion criteria
Potential subjects must meet all of the inclusion criteria, and none of the exclusion criteria, to be enrolled in this study.
DFU inclusion criteria
-
At least 21 years of age or older.
-
Diagnosis of type 1 or 2 diabetes.
-
At randomization, subjects must have a target ulcer with a minimum surface area of 1.0cm2 and a maximum surface area of 20.0 cm2 measured post-debridement.
-
The target ulcer must have been present for a minimum of 4 weeks and a maximum of 52 weeks, and treated with standard of care (SOC), before the initial screening visit.
-
The target ulcer must be located on the foot with at least 50% of the ulcer below the malleolus.
-
The target ulcer must be Wagner 1 or 2 grade, extending at least through the dermis or subcutaneous tissue and may involve the muscle provided it is below the medial aspect of the malleolus.
-
The affected limb must have adequate perfusion confirmed by vascular assessment. Any of the following methods performed within 3 months of the first screening visit are acceptable:
-
Ankle-brachial Index (ABI) between 0.7 and ≤1.3;
-
Toe-brachial Index (TBI) ≥0.6;
-
Transcutaneous oxygen measurement (TCOM) ≥40 mmHg;
-
Pulse volume recording (PVR): biphasic.
-
-
If the potential subject has two or more ulcers, they must be separated by at least 2 cm, post debridement. The largest ulcer satisfying the inclusion and exclusion criteria will be designated as the target ulcer.
-
Target ulcers located on the plantar aspect of the foot must be offloaded for at least 14 days before enrollment.
-
The potential subject must consent to using the prescribed offloading method for the duration of the study.
-
The potential subject must agree to attend the weekly study visits required by the protocol.
-
The potential subject must be willing and able to participate in the informed consent process.
DFU exclusion criteria
-
The potential subject is known to have a life expectancy of <6 months.
-
The potential subject’s target ulcer is not secondary to diabetes.
-
The target ulcer is infected, requires systemic antibiotic therapy, or there is cellulitis in the surrounding skin.
-
The target ulcer exposes tendon or bone.
-
There is evidence of osteomyelitis complicating the target ulcer.
-
The potential subject is receiving immunosuppressants (including systemic corticosteroids at doses greater than 10 mg of prednisone per day or equivalent) or cytotoxic chemotherapy or is taking medications that the PI believes will interfere with wound healing (e.g., biologics).
-
The potential subject is taking hydroxyurea.
-
The potential subject has applied topical steroids to the ulcer surface within 1 month of initial screening.
-
The potential subject has a previous partial amputation on the affected foot that results in a deformity that impedes proper offloading of the target ulcer.
-
The potential subject has glycated hemoglobin (HbA1c) greater than or equal to 12% within 3 months of the initial screening visit.
-
The surface area of the target ulcer has reduced in size by more than 20% in the 2 weeks prior to the initial screening visit (‘historical’ run-in period). Imaging Device is not required for measurements taken during the historical run-in period (e.g., calculating surface area using length X width is acceptable).
-
The surface area measurement of the target ulcer decreases by 20% or more during the 2-week screening phase: the 2 weeks from the initial screening visit (S1) to the TV-1 visit, during which time the potential subject received SOC.
-
The potential subject has an acute Charcot foot, or an inactive Charcot foot, which impedes proper offloading of the target ulcer.
-
Women who are pregnant or considering becoming pregnant within the next 6 months are excluded.
-
The potential subject has end-stage renal disease requiring dialysis.
-
The potential subject has participated in a clinical trial involving treatment with an investigational product within the previous 30 days.
-
A potential subject who, in the opinion of the investigator, has a medical or psychological condition that may interfere with study assessments.
-
The potential subject was treated with hyperbaric oxygen therapy (HBOT) or a CAMP in the 30 days prior to the initial screening visit.
-
The potential subject has a malnutrition indicator score <17 as measured on the Mini Nutritional Assessment.
-
A subject who has a wound with active or latent infection is excluded.
-
A subject with a disorder that would create unacceptable risk of post-operative complications is excluded.
-
A subject with a known sensitivity to aminoglycoside antibiotics is excluded.
VLU inclusion criteria
-
At least 21 years of age or older.
-
Diagnosis of type 1 or 2 diabetes.
-
At randomization, subjects must have a target ulcer with a minimum surface area of 1.0cm2 and a maximum surface area of 20.0 cm2 measured post-debridement.
-
The target ulcer must have been present for a minimum of 4 weeks and a maximum of 52 weeks, and treated with standard of care (SOC), before the initial screening visit.
-
No visible signs of healing objectively, less than 40% reduction in wound size in the last 4 weeks.
-
The affected limb must have adequate perfusion confirmed by vascular assessment. Any of the following methods performed within 3 months of the first screening visit are acceptable:
-
Ankle-brachial Index (ABI) between 0.7 and ≤1.3;
-
Toe-brachial Index (TBI) ≥0.6;
-
Transcutaneous oxygen measurement (TCOM) ≥40 mmHg;
-
PVR: biphasic.
-
-
If the potential subject has two or more ulcers, they must be separated by at least 2 cm, post debridement. The largest ulcer satisfying the inclusion and exclusion criteria will be designated as the target ulcer.
-
The potential subject must agree to attend the weekly study visits required by the protocol. • The potential subject must be willing and able to participate in the informed consent process.
VLU exclusion criteria
-
The potential subject is known to have a life expectancy of <6 months.
-
The potential subject’s target ulcer is not secondary to diabetes.
-
The target ulcer is infected, requires systemic antibiotic therapy, or there is cellulitis in the surrounding skin.
-
The target ulcer exposes tendon or bone.
-
There is evidence of osteomyelitis complicating the target ulcer.
-
The potential subject is receiving immunosuppressants (including systemic corticosteroids at doses greater than 10mg of prednisone per day or equivalent) or cytotoxic chemotherapy or is taking medications that the PI believes will interfere with wound healing (e.g., biologics).
-
The potential subject is taking hydroxyurea.
-
The potential subject has applied topical steroids to the ulcer surface within 1 month of initial screening.
-
The potential subject has a previous partial amputation on the affected foot that results in a deformity that impedes proper offloading of the target ulcer.
-
The potential subject has glycated hemoglobin (HbA1c) greater than or equal to 12% within 3 months of the initial screening visit.
-
The surface area of the target ulcer has reduced in size by more than 20% in the 2 weeks prior to the initial screening visit ('historical’ run-in period). Imaging device is not required for measurements taken during the historical run-in period (e.g., calculating surface area using length X width is acceptable).
-
The surface area measurement of the target ulcer decreases by 20% or more during the 2-week screening phase: the 2 weeks from the initial screening visit (S1) to the TV-1 visit, during which time the potential subject received SOC.
-
Women who are pregnant or considering becoming pregnant within the next 6 months are excluded.
-
The potential subject has end-stage renal disease requiring dialysis.
-
The potential subject has participated in a clinical trial involving treatment with an investigational product within the previous 30 days.
-
A potential subject who, in the opinion of the investigator, has a medical or psychological condition that may interfere with study assessments.
-
The potential subject was treated with hyperbaric oxygen therapy (HBOT) or a CAMP in the 30 days prior to the initial screening visit.
-
The potential subject has a malnutrition indicator score <17 as measured on the Mini Nutritional Assessment.
-
A subject who has a wound with active or latent infection is excluded.
-
A subject with a disorder that would create unacceptable risk of post-operative complications is excluded.
-
A subject with a known sensitivity to aminoglycoside antibiotics is excluded.
Subject exit/discontinuation criteria; DFU and VLU
-
The subject voluntarily withdraws from the study. • Subject death. • Subject acquires any of the listed exclusion criteria.
-
Subject completes the protocol (see Figure 3).
-
Subject is non-compliant with the protocol.
-
Subject's well-being, in the opinion of the investigator, would be compromised by study continuation. • Subject reaches the clinical endpoint of total wound closure (days of wound closure will be counted from the day of closure to day 84).
-
Subject experiences a protocol deviation that, in the investigator’s opinion, will compromise the subject's continuation in the study.
Study procedures
Informed consent
Subjects will be provided with an informed consent form (ICF) describing the study and providing sufficient information for subjects to make an informed decision about their participation. The consent form will be submitted with the protocol for review and approval by the Institutional Review Board (IRB) and the study's sponsor. The formal consent of a subject must be obtained before he/she is subjected to any study procedure. This consent form must be signed by the subject or a legally acceptable surrogate, and the investigator-designated research professional who obtained the consent. A blank copy of the IRB-approved ICF will be kept on-site and available to the investigator for review by interested parties.
Vulnerable populations
While vulnerable subjects will not be specifically recruited for this study, vulnerable subjects may be present in the potential subject pool. Additional procedures will not be required to ensure human subject protections for these subjects.
Randomization scheme
Subject randomization will be performed electronically in the electronic data capture system. Subjects will be randomized across up to 5 Intervention groups and 1 Control group per ulcer type. The randomization scheme for the Intervention groups is 1:1:1:1:1, with the Control group randomization 1.4:1:1 for the VLU Control and 1.7:1:1:1 depending on the number of Intervention groups enrolling in the study.
Given this is a platform study design, the study may be initiated with two Intervention groups and one common Control group. Following the first planned interim analysis, sample size may be adjusted to maintain power. Recruitment periods will overlap but will not be identical.
Laboratory testing procedures
Laboratory procedures for this trial include a urine pregnancy test for women of childbearing potential and a fingerstick HbA1c level during screening if necessary.
Clinical procedures
Clinical procedures are described for each clinic visit. Subjects will be seen at weekly intervals (± 3 days) for the 12-week treatment period. If additional dressing changes are required between the scheduled visits, they will be recorded as unscheduled visits; however, the subject assessment will be abbreviated for those visits. The study coordinator will note the reason for the unscheduled visit in the CRF.
Rescreening
If a subject initially fails to meet inclusion/exclusion criteria and is later reconsidered for participation, the subject will be re-consented and assigned a new screening number at the time of re-screening. Subjects who fail their first screening attempt may be re-screened again (i.e., up to three screenings total) and may be enrolled if they are found to meet all inclusion and no exclusion criteria at the second screening visit.
Screening visit: 14 days prior to enrollment
At the screening visit, informed consent will first be obtained from each participant. The investigator will then review the subject’s medical history to determine eligibility according to the study’s inclusion and exclusion criteria. Demographic information, including height, weight, body mass index (BMI), gender, and ethnicity, as well as medical and medication history, will be collected. All current medications will be recorded, including non-steroidal anti-inflammatory drugs (NSAIDs) and opioids. A vascular screening test will be performed unless results from the previous 3 months are available. Vital signs (blood pressure, pulse, and respiratory rate) will be recorded, followed by a general physical examination. The Mini Nutritional Assessment (MNA) will be administered, and hemoglobin A1c (HbA1c) will be obtained and recorded unless recent results (within 3 months) are available. For DFU patients, a Wagner Grade assessment will be conducted, and for VLU patients, the CEAP Classification will be performed. The Fitzpatrick Scale and a pain assessment using the Visual Analog Scale (VAS) will also be completed. Wound characteristics, including granulation tissue, non-viable tissue, depth, exudate, and periwound skin condition, will be documented. Historical wound measurements from 2 weeks prior to the initial screening visit will be obtained and recorded; during this period, digital planimetry is not required, and surface area may be calculated using length × width. If the wound size decreases by more than 20% compared to the historical measurement, the subject will be recorded as a screen failure and will not proceed to the screening phase.
Begin screening phase (14 days duration leading up to enrollment)
The screening phase, which lasts 14 days prior to enrollment, will follow the standard of care. Wound cleansing will be performed using normal sterile saline (NSS); antiseptics may be used during the historical and 2-week center screening periods but are not permitted during the treatment phase unless explicitly exempted. Sharp debridement will be performed to remove all non-viable tissue and slough. The ulcer will be photographed, and the surface area will be measured using a digital imaging device after debridement. A calcium alginate or foam dressing will then be applied; alternative dressings may not be used without approval from the medical monitor. For DFU patients, trial-specific offloading will be initiated for plantar or pressure-related ulcers. The preferred offloading method is the protective ambulatory brace; however, for patients unable to tolerate this, a total contact cast (TCC) is acceptable. All DFU patients must have a minimum of 4 weeks of documented offloading prior to enrollment in the trial.
TV-1 enrollment/randomization (treatment phase day 0)
14 days after the screening visit (± 3 days), the subject should meet all the inclusion criteria and none of the exclusion criteria. The subject should be assessed for adverse events, medications should be reviewed for any changes, a symptom-directed physical examination performed, and any changes should be documented. Additionally, the PAR should be reviewed for the 2 weeks of screening (must be less than 20%), vital signs and wound characteristics recorded, and the Forgotten Wound Scores (FWS) and Wound Quality of Life (wQOL) questionnaires administered. VLU subjects will be assessed using the Functional Ambulatory Category Scale (FACS). If the subject remains a candidate for the trial, continue with randomization; if they do not, it is recorded as a screen failure. Treatment is then administered according to randomization and performed (either SOC or SOC + treatment) as listed above.
TV-2 through TV-12, TV-13
During the treatment phase, from treatment visit 2 (TV-2) through treatment visit 12 (TV-12) or until wound closure, participants will undergo weekly visits that include both assessments and treatment procedures. At each visit, subjects will be assessed for adverse events, and their medication use will be reviewed, with particular attention to any changes in opioid or non-steroidal anti-inflammatory drug (NSAID) use. Vital signs will be recorded, and wound characteristics such as the amount of granulation tissue, non-viable tissue, depth, exudate, and the condition of the periwound skin will be documented. A pain assessment will be performed using the Visual Analog Scale (VAS). Additionally, FWS and wQOL questionnaires will be administered at TV-4, TV-8, and TV-12 (or the final visit if closure occurs earlier).
Treatment procedures during these visits include cleansing the wound with NSS; antiseptics are not permitted except under specific exemptions. Sharp debridement will be performed to remove all non-viable tissue and slough. Following debridement, the ulcer will be photographed and measured using the digital imaging device to determine the ulcer surface area. The ulcer will then be treated according to the participant’s assigned randomization arm and dressed with calcium alginate or foam dressings provided. No other dressings may be used without the prior approval of the medical monitor. For highly exudative wounds, additional absorptive dressings may be applied with the medical monitor’s permission. For DFU patients not using TCC, adherence to offloading will be assessed; note that only plantar ulcers require offloading with a protective ambulatory brace.
At treatment visit 13 (TV-13), or earlier if the wound closes prior to TV-12, subjects will again be assessed for adverse events, and any medication changes will be reviewed. Pain assessment using the VAS, as well as the FWS and wQOL questionnaires, will be completed. VLU patients will also undergo the FACS assessment. If the ulcer remains open, wound characteristics will be recorded. The ulcer will be photographed and measured with the digital imaging device. For subjects who have not achieved wound closure, appropriate follow-up wound care will be arranged. Finally, the end of study (EOS) form will be completed to document the participant’s study completion status.
Healing confirmation visit (2 weeks post-wound closure)
Healing is defined by the FDA as complete closure without drainage for 2 weeks. To meet this definition, the patient must return for a healing confirmation visit (HCV) 14 days (±3 days) after the first assessment of 100% reepithelialization. In the event that the first assessment of healing/closure occurs at end of study visit (i.e., end of week 12, TV-13), subjects will still return for a confirmation visit. The following procedures will be performed at the HCV: review of adverse events and concomitant medications, assessment of pain status, assessment of wound closure, wound imaging using the protocol imaging device, and confirmation of wound closure will be verified by independent staff blinded to study randomization.
Withdraw visit
If a subject withdraws early or the investigator terminates subject participation early, the procedures and data collection described for the final study visit (TV-13) will be performed.
Unscheduled visit
Subjects may require unscheduled (UNS) visits (e.g., for dressing changes). The study coordinator will complete the UNS case report form and document the reason for the visit. At an unscheduled visit, the following procedures will be conducted: review of potential adverse events, review of concomitant medications and replacement of secondary dressing, if required.
Follow-up procedures and therapy transitions
At the study's conclusion, if the wound has not healed, the subject will resume SOC treatments as prescribed by their physician. No additional follow-up procedures or transitions are required.
Independent confirmation of healing
In addition to the principal investigator’s determination of complete closure, an independent assessment of the primary endpoint will be performed. Wound care specialists not associated with the trial (‘the reviewers’) will review de-identified digital images of ulcers taken with a digital imaging device. Two reviewers who will be blinded to the arm of the study will assess each photograph and determine it as either ‘healed’ or ‘not healed’. If there is a disagreement between the reviewers, the reviewer who agrees with the principal investigator’s assessment will be used.
Data collection and analysis
Subject population(s) for analysis
The following patient populations are subject to study analysis:
-
Intent to treat population (ITT): Any subject who is randomized according to the randomization assignment.
-
All-treated population (mITT): Any subject randomized into the study that received at least one exposure to a study product.
-
Protocol-compliant population (PP): Any subject who was randomized and received the protocol-required study product exposure.
Statistical methods
Statistical methods will include descriptive statistics and hypothesis testing. Safety data (adverse events) will be compiled and compared between the interventional and control groups. Wound area measurements will be determined and compared over time to assess changes from baseline between interventional and control groups. Pain assessments will be compiled and compared over time to assess changes from baseline between the interventional and control groups. Days to wound closure will be compared between interventional and control groups. Missing data will not be imputed, and no attempt will be made to provide values for these missing data points. Planned analyses are listed in Table 1.
TABLE 1 Analysis plan
| Data Collected | Analysis to be Performed |
|---|---|
| Demographics data | Descriptive statistics; comparison to general population; significance at p<0.05 |
| VAS assessment | Descriptive statistics; Change from baseline over time in control and interventional groups; nominal p value will be provided |
| Wound measurements (area) | Descriptive statistics and hypothesis testing; area measurements change from baseline over time; comparison between groups, nominal p value will be provided |
| Adverse events related to the product | Descriptive statistics; total number of events over time; comparison between groups; nominal p value will be provided |
| Days to wound closure | Descriptive statistics; days to wound closure (out of 84-day study period) |
Continuous variables will be assessed for distribution and presented as mean (SD) or median with interquartile range (IQR). Categorical variables will use counts and percentages will be used to summarize data.
Primary endpoint
The primary endpoint is the proportion of target ulcers that achieve complete wound closure in 12 weeks. A Chi-squared test will assess this endpoint. The null hypothesis states that the proportion of wounds achieving complete wound closure in 12 weeks is equal to or less for multiple CAMPs plus SOC compared to SOC alone. The alternative hypothesis states that the proportion of wounds achieving complete wound closure in 12 weeks is superior for multiple CAMPs plus SOC versus SOC alone. The primary endpoint analysis will be based on the ITT population.
Secondary endpoints
To assess the secondary endpoints, a t-test will be performed. The following hypotheses will be tested:
H0: μ1– μ2 = 0
H1: μ1– μ2 = D1 ≠ 0
Where μ1 is the mean difference in area (cm2) for patients in multiple CAMPs plus SOC, μ2 is the same specified metric in SOC alone, and D1 is the difference (μ1– μ2) assuming the alternative hypothesis.
Where μ1 is the mean difference in VAS score for patients in multiple CAMPs plus SOC, μ2 is the same specified metric in SOC alone, and D1 is the difference (μ1– μ2) assuming the alternative hypothesis
Where μ1 is the mean difference in wQOL for patients in multiple CAMPs plus SOC, μ2 is the same specified metric in SOC alone, and D1 is the difference (μ1– μ2) assuming the alternative hypothesis.
PAR will be calculated using the formula below. Summary statistics will be calculated.
The number of adverse events, severe adverse events, and average number of grafts used will be reported using descriptive statistics by treatment group. All serious adverse events will be followed and reported.
Exploratory endpoints
Exploratory endpoints will be assessed using summary statistics and t-tests, chi-squared, Mann-Whitney, and other tests as appropriate.
All statistical testing will be two-sided with α = 0.05. The most recent version of R Studio (for Windows) and Python will be used for analysis. Two subgroup strata are planned; wound age and wound duration. Table 2 shows the planned stratification.
TABLE 2 Strata for randomization.
| Variable | Strata | Groups |
|---|---|---|
| Wound age | <60 days | 1. <2cm2wounds <60 days old 2. <2cm2wounds ≥60 days old 3. 2-3cm2wounds <60 days old 4. 2-3cm2wounds ≥60 days old 5. >3cm2wounds <60 days old 6. >3cm2wounds ≥60 days old |
| ≥60 days | ||
| Wound size | <2cm2 | |
| 2–3cm2 | ||
| >3cm2 |
Interim and final analyses
An interim analysis will occur when a minimum of 24 patients in each interventional arm have completed care (study products 1 and 2 for DFU and VLU), and a minimum of 32 subjects have completed SOC. During interim analysis, tests to determine if the treatment arms can be pooled will be conducted. If these tests are not significant, treatment arms will be analyzed individually against the SOC.
Treatment arms will be dropped if the mean percent area reduction (PAR) per treatment arm is less than 10% at the interim analysis. Should a treatment arm meet the removal threshold, an investigation will be conducted to determine if factors other than product performance impacted the clinical results.
The study will continue to enroll until the control arms reach approximately 70 patients based on the wound type control and the 5 interventional arms reach 40 completed patients each. Additional interim analyses may be conducted consistent with the adaptive design.
The number of adverse events and severe adverse events will be reported using descriptive statistics by treatment group. All serious adverse events will be followed and reported.
Anticipated benefits and risks/risk mitigation
The benefits associated with this study are that investigators will gain insight into the safety and performance of the CAMPs being evaluated. Risks associated with ulcer care related to this study include ulcer management and wound cover risks.
Potential risks associated with study procedures are listed in Table 3 along with the applicable risk mitigation measures.
TABLE 3 Potential risks and risk mitigation.
| Study procedure | Anticipated risks | Risk mitigation |
|---|---|---|
| Wound debridement | Pain | Procedures to be performed by trained clinical staff |
| Wound measurements | None anticipated | N/A |
| Pain assessments | None anticipated | N/A |
| Wound photos | None anticipated | N/A |
| CAMP/skin substitute application | This allograft has the potential to transmit infectious disease to the recipient Potential allergic reaction/skin irritation |
Strict donor screening and laboratory testing, along with dedicated processing and sterilization methods, are employed to reduce the risk of any disease transmission. However, as with all biological implants, an absolute guarantee of tissue safety is not possible Patients with a known sensitivity to aminoglycoside antibiotics are excluded from participating in the study |
| Dressing placement | None anticipated | N/A |
| Total contact casting | None anticipated | N/A |
| Ankle-brachial Index (ABI) | Discomfort in area of skin breakdown secondary to pressure from cuff | Topical lidocaine |
Discussion
DFUs and VLUs represent a significant global health burden, affecting a substantial proportion of individuals with type 2 diabetes and contributing to increased morbidity, mortality, and healthcare expenditures. Despite the availability of established SOC practices, including sharp debridement, pressure offloading, infection control, and moisture management, clinical outcomes remain suboptimal. Fewer than half of DFUs achieve closure within 12 weeks of treatment initiation. These healing challenges are compounded by socioeconomic disparities in access to advanced wound care, underfunding of chronic wound research, and a scarcity of high-quality comparative effectiveness data to guide therapeutic decision making.
In response to these unmet needs, interest in the use of CAMPs has increased. These advanced wound care products have demonstrated favorable biological activity in preclinical and early clinical studies and may enhance healing in complex, non-healing wounds. However, their evaluation has been limited by the constraints of conventional randomized controlled trials, which often assess single products in isolation, without accounting for evolving clinical standards or permitting comparative assessment of multiple interventions within a consistent framework.
Conclusion
This trial is expected to provide high-quality efficacy data on CAMPs and contribute to evidence-based practice in DFU and VLU management. The modified platform design offers a flexible and efficient approach to evaluating multiple interventions within a single clinical trial.
Future research may expand on this platform to include additional product classes, explore subgroup analyses based on wound characteristics or patient risk profiles, and incorporate emerging digital technologies for wound monitoring. The successful implementation of this trial will add to the fund of knowledge regarding the utility of modified master protocol designs, provide a trial model for future investigations in regenerative medicine and support the evolution of more responsive, efficient, and evidence-based approaches to chronic wound management.